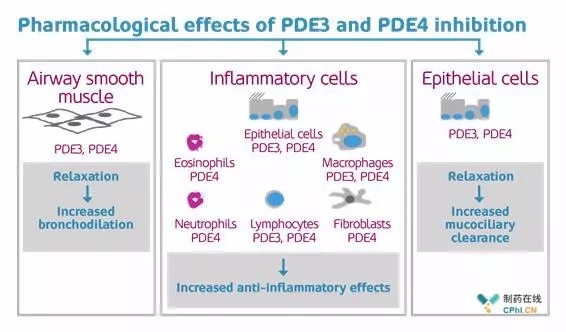
Verona's new drug RPL554 has an excellent cystic fibrosis IIa test, and its share price has risen by 20%! March 5, 2018 Source: CPhI Pharma Online Author: Knowing and Doing On March 2, 2018, Verona Pharma announced positive results in its Phase IIa clinical trial of the new drug RPL554 for the treatment of cystic fibrosis (CF). Forced expiratory of single-dose RPL554 compared to baseline 1 second. Volume in one second, FEV1) increased by 6% (P < 0.05) to achieve the expected results, Verona's share price rose 20% that day. CF is the most common lethal hereditary disease in the United States and Europe, and it usually causes impaired lung function in patients and is recognized as a rare disease by the FDA. Because the mucus secreted in the lungs of CF patients is too thick, on the one hand, it can not play a normal role, on the other hand, it will frequently and continuously infect bacteria. CF is caused by the absence of exon of the cystic fibrosis transmembran conductance regulator (CFTR) gene. There is no treatment for CF, and frequent hospital treatment has severely reduced patients. The quality of life, the average age of death for CF patients is 40 years. There are 70,000 CF patients worldwide, compared with 42.9% in the United States and 1,000 new patients each year. The innovative drug RPL554 developed by Verona Pharma is a dual inhibitor of phosphodiesterase 3 (PDE3) / phosphodiesterase 4 (PDE4), an aerosol spray anti-inflammatory drug and bronchodilator, developed in Verona's research and development pipeline. RPL554 is currently in clinical trials for Phase II first-line medication for chronic obstructive pulmonary disease (COPD) and CF, and will be used in the treatment of asthma. RPL554 is designed to maximize efficacy while reducing side effects. As a dual inhibitor of PDE3/PDE4, the PDE4 inhibitor used in RPL554 is chemically structurally distinct from traditional PDE4 inhibitors (avoiding related side effects such as gastrointestinal) At the same time, the high screening of PDE3/PDE4 by RPL554 reduces its off-target effect. For respiratory diseases, RPL554 mainly acts in three aspects: soothing the respiratory muscles, increasing bronchodilating; acting on inflammatory cells to enhance anti-inflammatory effects; acting on epidermal cells to accelerate the clearance of mucociliary cilia. According to Verona, RPL554 statistically showed a significant enhancement of lung function compared to placebo in early clinical trials. Mechanism of action of RPL554 (PDE3/PDE4 double inhibitor) (from Verona Pharma's official website) The primary objective of the IIa clinical trial published by Verona on March 2, 2018 was to evaluate the tolerability of RPL554 in CF patients and its PK/PD (pharmacokinetics/pharmacodynamics). This randomized, placebo-controlled group used 1.5. The therapeutic doses of mg and 6.0 mg rose by 20% on the day of the test results. The trial data showed that the primary endpoint of PK/PD was consistent with RPL554 in patients with COPD, with a half-life of 7.5 h and 10.1 h at 1.5 mg and 6.0 mg, respectively. The secondary endpoint, FEV1, reached or exceeded Verona's expectations, exceeding 4% (P < 0.05) and 6% (P < 0.05) of baseline at 1.5 mg and 6.0 mg, respectively, indicating that anti-inflammatory drugs and bronchodilators were mixed. A spray formulation, RPL554, may improve the quality of life of patients with CF. "A single-dose increase of 6% of FEV1 compared to baseline is a critical outcome for CF patients," said Dr. Andres Floto, chief inspector of the trial. However, we have to notice that this IIa clinical trial is a small-scale (10 patients), short-term trial, and the more detailed clinical results have not been announced, the results of Verona at this stage have yet to be further verified. The IIa clinical trial is an exploratory study. The main purpose is to study the dose range of the drug and PK/PD. The clinical trial of IIb will further study its effectiveness and safety. Verona's follow-up IIb clinical trial on RPL554 will be carried out as soon as possible. And announced. Jan-Anders Karlsson (from Verona Pharma's official website) Dr. Jan-Anders Karlsson, CEO of Verona, agrees with the results of this trial and is looking forward to the IIb test data for COPD of RPL554. "This trial data supports the development of RPL554 in CF treatment. We look forward to the announcement of 2018Q2. RPL554 results in clinical IIb top-line testing for maintenance of COPD and further optimizes the future development of RPL554." Reference source Verona jumps as midphase cystic fibrosis data dial up hopes; 2. Verona Pharma's official website. We're professional Lithotriptoscopy Set manufacturers and suppliers in China, specialized in
providing high quality medical instruments with reasonable price. We
warmly welcome you to buy or wholesale bulk Lithotriptoscopy Set
for sale here and get quotation from our factory. Lithotriptoscopy Set,Urology Urethrotomy Set,Surgical Lithotriptoscopy Set,Endoscopic Lithotriptoscopy Set Tonglu WANHE Medical Instrument Co., Ltd , https://www.tlvanhurhealth.com