Several mutations know the efficacy of new blood tests can predict whether advanced immunotherapy can fight cancer July 10, 2018 Source: Chinese Journal of Science and Technology Immune cells (circles) or more susceptible to the presence of many tumor cells that alter the mutation of the protein. Image source: STEVE GSCHMEISSNER Some cancers produce seeds of self-destruction. Certain random mutations that accumulate in rapidly dividing tumor cells can stimulate the immune system to attack such cancers. Researchers now understand that this degree of mutation predicts whether a cancer will respond to powerful, immune-based new therapies. A recently published blood test for this tumor mutation burden (TMB) may help to become a useful tool for guiding cancer treatment. Cancer researchers have been able to detect TMB by sequencing a selected set of genes in biological living tissue, a method that has recently shown strong predictive power in large lung cancer trials. Some cancer doctors have now implemented tissue TMB testing in some cases. Minimally invasive blood testing for the analysis of exfoliated tumor DNA in the human blood circulation, or the discovery of TMB in many patients who do not contribute to tissue testing. Naiyer Rizvi, an oncologist at Columbia University Medical Center, said: "We will see more and more TMB." Nevertheless, he added that TMB testing currently takes too long in daily clinical practice, some in cancer research. People question how much it will ultimately be useful. It is urgently needed to predict whether immunotherapy can function in patients, especially for checkpoint inhibitors, which will inhibit the release of immune cells and cause them to attack tumors. Since the US Food and Drug Administration (FDA) approved the first antibody drug targeting the "checkpoint" protein PD-1 in 2014, these drugs have changed cancer treatment. UCLA oncologist Antoni Ribas pointed out that by May of this year, half of cancer patients in his hospital had been taking checkpoint inhibitors for the past six months. "We are using these drugs at very high rates, which should be noticed," he said. Some patients responded very significantly, but most people still failed to benefit, while others never took the drug. In addition to 4% of patients with specific DNA repair defects in the tumor, the doctor is not sure who will benefit from it. Thus, TMB detection is coming. Most analyses estimate the number of mutations in a tumor that alter the protein by sequencing a limited number of genes in the tumor DNA; this data may reflect the density of the mutein fragment (ie, the new antigen) on the surface of the cancer cell. These fragments do not help tumor growth; they are just a by-product of the error-prone tumor cell division. But they are indeed foreign to the immune system - the more new antigens, the more likely immunotherapy is to shrink tumors and inhibit their growth. In a lung cancer trial reported at the annual meeting of the American Association for Cancer Research (AACR) in Chicago, Illinois in April, the researchers found that the mutation load in tumor tissue predicts whether a combination of checkpoint inhibitors can help lung cancer better than conventional chemotherapy. patient. More than 40% of lung cancers show a higher TMB, and on average, patients with such tumors perform better in immunotherapy. Rizvi said the Phase III trial of 1,739 patients will be approved by the FDA, which was developed by the Basic Medicine Company of Cambridge, Mass., for lung cancer treatment. (In mid-June, Swiss pharmaceutical giant Roche has promised to acquire the company) At the annual meeting of the American Society of Clinical Oncology (ASCO) in Chicago in June, more evidence showed the predictive value of TMB. David Gandara, an oncologist at the University of California, Davis, reported a retrospective analysis of seven different trials of the checkpoint inhibitor Tecentriq in lung cancer, bladder cancer, melanoma, and other tumors. As the same tissue test shows, when TMB is higher, the tumor responds to the drug at a multiple rate. "The future of TMB is now open," Gandara said at the ASCO conference. However, organizing TMB testing is "very expensive. It requires a lot of organization and is not standardized." Yale University pathologist David Rimm said. In the trials reported at the AACR meeting, doctors could only get enough tumor tissue from 58% of patients. Rizvi added that the entire process may take up to three weeks, and the waiting time for newly diagnosed patients is too long. Blood TMB tests, also from basic medical companies, may prove to be as effective as tissue testing. At the ASCO meeting, Vamsidhar Velcheti of the Cleveland Clinic in Ohio reported preliminary results of a prospective trial of Tecentriq in lung cancer patients undergoing TMB blood testing. The drug reduced high-mutation burden tumors by more than 36%, but only 6% for low-TMB tumors. Patients with high TMB tumors have twice the time to relapse from cancer than patients with low TMB tumors. But Hossein Borghaei, an oncologist at the Fox Chase Cancer Center in Philadelphia, Pennsylvania, warned that Velcheti only reported the first 58 patients. Currently, another trial, including 580 patients, is underway. Rimm agreed that the initial results need to be verified. In April of this year, the FDA considered the blood TMB test to be a “breakthrough device†worthy of priority evaluation. But whether it's a blood test or a biopsy, it's unclear whether TMB will give doctors and patients the results they desire. Rimm noted that trials have not shown that patients with high TMB receive immunotherapy longer than chemotherapy. Ribas predicts that TMB will be an integral part of future composite biomarkers. (Jin Nan compiled) Chinese Journal of Science (2018-07-09 3rd Edition International) Herbal Extract
Herbal extract is a concentrated solution made by extracting the herb's chemical constituents out of the inert herb fiber (cellulose) with a solution of alcohol and water or glycerin. This process allows the beneficial components of plants, such as antioxidants, vitamins, and minerals, to be extracted and used for medicinal or therapeutic purposes. Commonly used herbs include echinacea, ginseng, turmeric, and milk thistle. Herbal extracts can be consumed in various forms, such as capsules, tinctures, or teas.
According to different extraction methods: the volatile oil, oil, extract, fluid extract, dry extract, active components and active parts obtained from plants for preparation production are all extracts.
Herbal Extract,Herbal Extract Powder,Herbs Extract Shaanxi Zhongyi Kangjian Biotechnology Co.,Ltd , https://www.zhongyibiology.com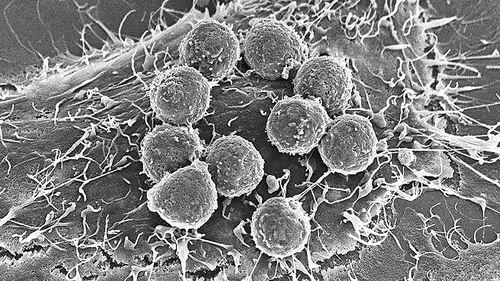
Herbal extracts are classified according to their efficacy:Immune-boosting extract,Antialcoholic Liver Extract,Sleep Improving Extract,Purgative Extract,Treat Pharyngeal Extract.
According to the characteristics of the final product, it can be divided into oil, liquid, powder, lens and so on.