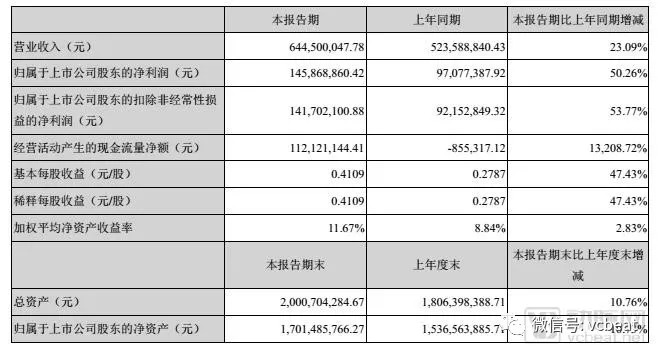
On the evening of August 21, 2018, the genetic testing giant Berry Gene released the financial results for the first half of 2018. Image taken from Berry Gene 2018 semi-annual report Berry Gene is a research and development biotechnology company dedicated to the application of high-throughput gene sequencing technology to provide "non-invasive" total solutions for clinical medical disease screening and diagnosis. It is also an industry leader in clinical transformation of gene sequencing technology. . The semi-annual report shows that in the first half of 2018, Berry's gene sales revenue was approximately 645 million yuan, up 23.09% year-on-year; net profit attributable to listed company shareholders was approximately 146 million yuan, up 50.26% over the same period. . The previously disclosed Q1 financial report shows that in the first quarter of 2018, Berry's gene realized revenue of approximately 297 million yuan, a 32.81% increase over the same period of the previous year; the net profit attributable to shareholders of listed companies was approximately 65.67 million yuan. It increased by 69.37% year-on-year. The comparison of the two sets of data shows that Berry's genes have shown a good momentum of sustained and steady growth in the first half of the first half of the year. Image taken from Berry Gene 2018 semi-annual report Part of the increase in performance was due to the overall increase in the market, and the other part was due to technological innovation and recognition of the company by the user market. In addition to the upstream instrument consumables and midstream testing services, Berry's investment in the Big Data Industrial Park has also officially started. In addition, the arterial network also noted that Berry's gene in a low-key in June 2018 invested in a gene therapy company in Suzhou - Faith Medicine. This is a bio-based company that specializes in the research and development and industrialization of gene therapy drugs and their carriers. Undoubtedly, this company has an upstream and downstream synergy in the main business of Berry Gene, genetic testing. This is also the first time that Berry's gene is laid out downstream of the entire industry chain of gene sequencing. The idea that Berry's gene first revealed its industrial layout to the arterial network was in 2016. Today, the so-called industry is not just a simple sequencing industry. What is the whole industry layout, is it the whole industry chain related to gene sequencing, or is it genetically related? How will they plan and lay out step by step? When did the idea of ​​the whole industry layout sprout? “When the company was first established in 2010, we actually had a 'full industry concept.' It’s definitely not going to be a link.†Zhou Daixing told the arterial network, “When the NIPT business is ready to begin to leap, we will What else can we do after thinking about NIPT?" Berry Gene Co-founder Zhou Daixing With the problem, the arterial network was linked to Dr. Zhou Daixing, the co-founder of Berry Gene. In the following hour, he introduced the logic of Berry's gene in the whole industry to the arterial network. What is included in their industrial layout? And how to implement it step by step in the future. The layout of the upstream market began in 2015 and has now entered nearly 100 hospitals across the country. In 2015, Berry Gene and Illumina jointly developed the second-generation sequencer NextSeq CN500, which is dedicated to China's clinical development, for the first time in the upstream instrument market. After several years of development, NextSeq CN500 has entered nearly 100 hospitals across the country, occupying 70% of the market share of the product business model in this market, and has become the largest sequencing company in the world. Berry's gene has also introduced sequencing platforms such as PacBio, Nanopore, and 10X to continuously increase the strength of scientific research services. As part of the upgrade to existing inspections, the other side is also verifying the potential for more applications of these sequencing platforms in China. Midstream: Tumors and genetic diseases are the core In the midstream, the Berry gene currently puts the center on the detection of tumors and genetic diseases. Relying on the nationwide sales network and a number of medical laboratories, Berry Gene extends these two lines of business to cities across the country. The genetic disease business represented by NIPT is the main business of Berry Gene. The corresponding testing services and kits and equipment sales are the current sources of cash flow. After years of hard work, they have taken an absolute advantage in the field of non-invasive prenatal testing, and the world's first large-scale clinical case return of more than one million samples. Currently, NIPT's detection range only covers chromosome aneuploidy (T21, T18 and T13) detection. But there is nothing wrong with it, extending to more elaborate chromosomal structural variations—chromosome microdeletions and micro-repetitions are inevitable trends. As a pioneer in the non-invasive prenatal field, the larger detection range and more accurate test results are also the continuous investment of Berry Gene. At the end of 2015, the NIPT Plus project technical filing application jointly developed by Berry Gene and Hunan Jiahui Genetic Specialist Hospital was approved by the Hunan Provincial Health Planning Commission. This is the only NIPT Plus product approved in China. Subsequently, Berry Gene was the first to introduce the NIPT Plus product covering 14 chromosomal diseases, Bebean Plus, and completed a 100,000+ sample return in just two years. In January 2018, after two years of validation, Berry Gene officially upgraded the NIPT detection range to 100 chromosomal diseases. The oncology business is another focus of the company before and after non-invasive production, and it is also the next market that is about to break out. Berry's first introduction of tumor detection products was launched in 2015, followed by cncART as the core technology of Onconi tumor molecular diagnostic products. But the idea of ​​the first to enter the tumor business was in 2012. Berry's technical team started with non-invasive chromosomal disease testing, and naturally expanded to non-invasive prenatal testing of single-gene disease. Shortly after the company was founded, the company began researching the technology of non-invasive prenatal single-gene disease, the later cSMART technology. Later story development tells us that cSMART technology laid the foundation for the development of Berry's gene tumor business. This technique detects very low levels of fetal DNA mutations from maternal plasma. In the same way, it can also be used for the detection of tumor DNA. After all, the free tumor DNA content in the peripheral blood of tumor patients is also very low. In July 2018, cSMART liquid biopsy technology, known as the “revolutionary breakthrough in molecular diagnosisâ€, obtained Chinese patents, which once again consolidated the core competitiveness of Berry Gene in the field of cancer. The Berry gene shows its determination to make a tumor business, starting with the establishment of a subsidiary and Rui Gene. Berry Gene established a subsidiary company and Rui Gene in August 2017, and in November of the same year, seven investment institutions, including Junlian Capital, invested a total of 800 million yuan in the company. The Swiss gene is based on the Berry gene, just like Grail is in Illumina. But slightly different, and Ruirui took over the parent company's full-line cancer business. After the capital increase and share expansion, Berry Gene retained the priority of the subsidiary's share repurchase. In April 2018, He Rui Gene United Nations Liver Cancer Science Center / Naval Military Medical University Eastern Hepatobiliary Surgery Hospital, Southern Medical University Southern Hospital jointly launched a national multi-center, prospective population cohort liver cancer early warning markers screening project. This plan marks the pioneering entry of China's liver cancer prevention and control into the very early stage of prevention and control and clinical verification, which is expected to greatly improve the diagnosis of liver cancer, especially early liver cancer. "Liver cancer is a unique 'large cancer type' in China, and it is also the most popular project in the early diagnosis industry." Asked and chose to use the liver cancer project as the starting point for the early screening business, Zhou Daixing answered this question. Liver cancer is a hidden disease, and patients rarely feel any discomfort in the early stages of the disease. This causes the disease to usually enter the advanced stage once it is discovered, and there is almost no treatment. “The 5-year survival period will not exceed 10%,†he added. Unfortunately, China is also a big country with liver disease, with more than 45 million people at high risk of liver cancer. These people are usually cirrhotic, liver fibrosis patients, about 1% of each year will develop into liver cancer, and its incidence is even higher than hereditary diseases. Therefore, for this part of China's patients, it is the best solution to use reliable technology to detect disease screening when early tumor cells do not spread. Because they can also control the condition through treatments such as surgery and chemotherapy, and win the best treatment period. "We discussed with many industry experts when we decided to do this, and this is the direction they have been seeking." Zhou Daixing said, "In addition, we have also made a long-term technical reserve, which is technically very confident." Downstream: What can I do after the data is generated? The upstream and midstream markets are actually producing data. To make the data known and used by people, it depends on the downstream interpretation and analysis of the data. This is the area that Berry has been focusing on since its launch. 1. Gene big data, an emerging market "After the test, a lot of data will be generated, and the amount of this data will be amazing in the future." Zhou Xingxing told the arterial network. Genetic testing begins with non-invasive prenatal, promising tumors, and will be in full-genome testing in the future. If everyone conducts a full genetic test after birth, these data are collected to form a big data market. The acquisition, management, storage, interpretation and retrieval of data will place very high demands on data processing capabilities. “This is a market we think is emerging,†he added. The current gene big data is still in a fragmented state, and has not yet formed a link in the industrial chain. However, he stressed that from the current phenomenon, the improvement of data processing and storage capacity will become an essential foundation for the next step of competition in the industry. Berry Gene invested in the establishment of the Gene Data Industrial Park in Fujian in 2017, focusing on the gene big data industry. It is worth mentioning that the industrial park is based on the big data of the Chinese population's disease-causing gene information database. Through cloud computing, gene sequencing, gene editing, artificial intelligence and other technical means, it covers the production, learning, research, and The four major sectors of the ecosystem, the goal is to establish China's first complete life sciences industrial cluster. In April 2018, the first phase of the Gene Big Data Center Industrial Park, which was brewing for 7 months, was officially started. According to the semi-annual report, the first phase of the Industrial Park project mainly includes research and development and industrialization projects for the early diagnosis and treatment of cardiovascular diseases and extracellular free DNA protection technology. "We have done a lot of early hardware investment in the industrial park, and the database of Chinese genetics has been out of scale." Zhou Xingxing revealed to the arterial network. Not only that, Berry Gene also introduced Professor Shen Yiping from Harvard University to help the company improve its interpretation and consulting skills. At the same time, it also introduced a welfare professor from the medical school to lead the construction of the entire information team. The big data market is ready. 2. What is after the diagnosis? treatment Of course, the entire industry chain envisioned by Berry Gene does not stop there. “What can we do with this data?†After the industry quickly took off and developed, Zhou Xingxing asked himself this way. If someone is diagnosed with a disease, the next step is definitely treatment. Beginning with genetic diseases, many companies have begun to use genetic technology to conduct research on the treatment of certain diseases, such as hemophilia, tumors, thalassemia and so on. Among them, the uselessness of tumors is a research hotspot. Two of the three gene therapies approved by the FDA in 2017 are related to tumors. "If it is only a diagnosis, there must be restrictions on the development of the industry in the future," he said. He believes that gene therapy is a rapidly emerging and promising treatment. "This is closely related to our big data and genetic testing, which can form a synergy between upstream and downstream." Zhou Xingxing added. He also revealed that in the future, Berry Gene will look for opportunities to collaborate with more excellent gene therapy companies and even establish their own gene therapy divisions. "Now we are choosing to invest in some gene therapy related companies from the beginning of investment." Zhou Daixing said. In June 2018, Berry Gene and Sherpa initiated a round of financing for Suzhou Faith Medicine, officially taking the first step from diagnosis to treatment. 3, the consumer market is promising In addition to treatment, there is a consumer business associated with big data. "In the Chinese market, everyone's understanding of consumer genes is still relatively small. The European and American regions have been rising for many years." Zhou Daixing said. In addition to the ancestral detection represented by 23andMe, consumer-grade genetic testing also contains other very large content. For example, the individual's nutritional metabolism, such as the lack of an enzyme, leading to poor intake of certain vitamins and so on. "Around the nutrition, in addition to the detection, after the test, it can also form a system service around the detection." He explained. For example, the motion gene detection represented by DNAFit is used to detect whether the user is suitable for high-intensity or mild exercise by detecting the genes related to exercise ability. And this data can help users or their fitness instructors to develop a more appropriate exercise program. During exercise, the amount of DNA in the blood also changes, and the genetic information displayed by these changes can also help users adjust their exercise habits. “These are all consumer-grade genetic markets,†he concluded. Interestingly, consumer-grade genetic testing is also the direction that giant Illumina has always been optimistic about. In addition to the non-invasive prenatal and early tumor screening businesses, they also set up Helix, a subsidiary of consumer-grade genetic testing. In addition to the non-invasive prenatal business of the main body of the Berry gene, the subsidiary and Rui Gene are just against Grail. The reply from Zhou Daixing also shows that the company is optimistic about the consumer-grade gene market. This will inevitably make people curious. In the future series of Berry Gene, will there be a subsidiary of Helix? “There is no action in the consumer market for the time being, but there will definitely be in the future,†he said. Investing in the most socially demanding places Upstream instrument consumables, midstream testing services, downstream big data, gene therapy and consumer industries, this is the description of Zhou Zhouxing's entire industry in an hour of interviews. Under the current development situation, the midstream market is undoubtedly the most invested part, but from the perspective of the company's attention and investment in other links over the past six months, the downstream layout is in full swing. “The focus is on the size of the market. The biggest focus of the current stage must be the detection of genetic diseases.†He said, “Investment is also the biggest.†In the next stage, big data and consumer-grade genetic testing are likely to be the next focus. At this stage, Berry's investment in big data is basically in the stage of hardware and interpretation. The big data market has not really emerged, and there is no consumer demand for data storage from the company. But what is certain is that with the development of the industry, the era of big data will finally come, and it will become a barrier for the genetic testing industry to continuously break through. In the consumer sector, the industry generally believes that this will be a huge market. According to a BCC survey, the consumer-grade testing market has been sailing at more than 70% in recent years, and its market share is expected to reach $240 billion in a few years. “We will focus our investment on the areas with the greatest social needs and make the most important investments in the most important places at the most needed time.†He added, “The maturity of technology will also have an impact.†From diagnosis to treatment, the trend of genetic technology development at home and abroad is sunny Half a month before Berry’s semi-annual report, Illumina also released its 2018 Q2 quarterly earnings report. The financial report shows that Illumina's total revenue for the second quarter of 2018 was $870 million, and revenue increased by 25% year-on-year, exceeding analysts' estimate of $786.6 million. Among them, the revenue of sequencing consumables was strong, reaching 455 million US dollars, exceeding the company's forecast. In addition, the overall revenue of the microarray business increased by 25% year-on-year to reach $140 million. The large-scale population genome project and the growing consumer-grade genetic testing market have helped the company's sequencing and microarray business to improve. But look at Natera's data. The fourth-ranked company in the non-invasive prenatal field in the United States had a total revenue of $63.07 million in the second quarter of FY18, up 21% year-on-year, and net profit was $2.72 million, down 4.22% year-on-year. The data of the two companies also seems to confirm that Zhou Daixing said, "Only one link will not work." Perhaps it is this understanding, Berry Gene has a sense of urgency when the non-invasive production of silk screen business is taking off, began to lay out a more extensive business sector. Of course, in addition to the two companies mentioned above, the profits of foreign companies such as Thermo Fisher, Agilent, Qiagen and PacBio have risen to varying degrees. Whether it is domestic or foreign, genetic sequencing technology has been developed. Not only that, 23andMe's disease risk assessment service was lifted; the US FDA approved the large panel test of MSK and Foundation Medicine. These represent the trust and recognition of the technology at the regulatory level, and it also indicates that it will occupy an increasingly important position in medical health and health management. The gene therapy of Novartis, Kite and Spark Therapeutics has been approved, which marks a leap from the diagnosis and treatment of gene technology. In China, following the NIPT, the Food and Drug Administration has approved the NGC-based NGS-based oncology diagnostic (tissue biopsy) kit. This is a major breakthrough in the domestic regulatory level. “It shows that the regulatory authorities have already done enough understanding of this industry, which is very good news for the industry.†Zhou Daixing believes. This also indicates that the regulatory authorities are facing the first opening of other testing services other than NIPT. In the future, there will be more companies to declare products, and more and more products will enter the clinic. We can imagine that the market for cancer clinical testing is being opened step by step, the cost is falling, and the maturity of technology and market brings more possibilities. And with it, it will be the consumer market, the big data market has exploded. In this context, companies with independent intellectual property rights and forward-looking awareness represented by Berry will usher in more opportunities.
In hip joint replacement surgery, can be broadly divided into two kinds,cemented artificial hip and cementless artificial hip, due to the selection of different materials, the price is also very different.
Total cementless hip system indications
1.The main purpose of total hip replacement is to relieve hip pain,and the second is to improve hip function. The main indication for total hip arthroplasty is hip pain caused by hip lesion over 60 years old,which can not be used for other operations but only for head and neck resection.
2.Replacement surgery may be considered in patients over 45 years of age with subluxation of the hip,traumatic arthritis, pain, or loss of function.Due to the insufficient depth of the acetabulum,the upper margin of the acetabulum is inclined, which affects the stability of the acetabulum cup,and the acetabulum should be deepened or covered.
3.Indications of the need for replacement include hip pain due to loosening of the prosthesis,stem fracture,prosthesis dislocation, manual reduction failure,central dislocation caused by acetabulum wear caused by prosthesis and pain.
hip joint,hip replacement,total hip replacement,hip surgery Jiangsu Aomed Ortho Medical Technology Co.,Ltd , https://www.aomedortho.com

Generally , the cemented type is suitable for osteoporosis, poor bone conditions, 65 to 70 years of age and older patients. The use of bone cement for artificial hip joints allows for early mobility, which is also suitable for older patients. In addition, elderly patients are generally less active, artificial joint wear is also light, replacement of an artificial joint can be, generally need less revision. The price of bone cement type artificial joint is low.
Cementless type is also known as biological fixation type, suitable for young and middle-aged patients with good bone conditions, such artificial joints have micropores or biological coating materials on the surface, bone can grow into it to achieve the role of fixing artificial joints, so it is often called biological fixation type. Cementless artificial hip joints allow their bones to slowly grow together with the artificial joint to achieve a fixed role.