Drosophila "send" anticancer drugs: reveal the story behind the birth of anticancer drugs April 17, 2019 Source: Singularity Network Sometimes, there seem to be inextricable links between two things that seem to be without aside, such as the unloved small fruit fly and the anti-cancer PARP inhibitor. This subtle connection began with an accidental discovery more than 90 years ago. Great prophecy In 1922, Calvin Bridges, a geneticist at Columbia University in the United States, discovered an interesting phenomenon when studying the hybridization of Drosophila melanogaster: Drosophila with two specific gene mutations could not survive, and when these two genes were mutated separately At the time, it will not cause fatal damage to fruit flies [1]. More than 20 years later, Theodore Dobzhansky, who worked at Columbia University, once again found a similar phenomenon in the Drosophila melanogaster, and officially named it "synthetic lethal" [2]. This idea was almost forgotten in the next half century until the emergence of Stephen Friend at the Fred Hutchinson Cancer Research Center. In 1997, Friend boldly predicted in "Science" that the concept of "synthesis to death" can be used for the development of anticancer drugs [3]. Friend's conjecture is not unfounded. You should know that there are a lot of genetic mutations in cancer cells. If you can find two genes that can simultaneously perform similar biological functions, if one of the genes is mutated, then the drug will be used to lose the function of the other gene. Achieve the effect of "synthetic death"! Although the truth is so simple, it takes a lot of hard work to turn the prophecy into reality. At this point, the main location of the story is to be transferred to Europe on the other side of the Atlantic. Looking for 觅觅 First came the French biologist Pierre Chambon. In 1963, Chambon inadvertently discovered an enzyme with DNA polymerization activity during the study of RNA polymerase using chicken liver nucleic acid extract [4], which is PARP (poly ADP ribose polymerase). However, Chambon is still addicted to the research of RNA polymerase, and soon PARP will be left behind. At this time, a group of British scientists began to show their talents. First in 1971, JB. In order to explore the function of PARP, Clark et al. first found a substance that inhibits PARP activity, nicotinamide [5]. In a few years, Barbara Durkacz et al. demonstrated for the first time that nicotinamide analogues (3-aminobenzamide, 3-AB) inhibit DNA repair and enhance the cytotoxicity of the DNA damaging agent dimethyl sulfate [6] ]. However, 3-AB is not selective and can only exert its inhibitory activity against PARP at a millimolar concentration. This means that even experimenting with animals does not work, let alone for clinical trials. However, this has greatly stimulated the interest of scientists, and a large number of scientists have devoted themselves to the screening and exploration of PARP inhibitors. They are constantly looking for more active and selective PARP inhibitors. At the same time, scientists have gradually figured out the function of PARP. It turns out that PARP is a big family. To date, 17 PARPs have been discovered [7], of which PARP-1 plays a key role in DNA single-strand break repair. PARP-1 can accurately recognize and bind to the gap of DNA single-strand break, while the surrounding nicotinamide adenine dinucleotide (NAD+) will rapidly form a complex with the active site of PRAP-1, and this complex will be called to other The effector molecules involved in DNA repair quickly fill the gap in DNA fragmentation [7]. After completing the DNA repair, PARP-1 is like a fameless hermit, detached from the DNA, returned to the free state, waiting for the next mission to repair single-strand breaks [7]. In addition, PARP-2 in the PARP family can accurately identify single-strand breaks, but it is more like a reserve army, because PARP-1 plays more than 90% of the functions in repairing DNA damage [8]. For PARP-3, which also plays a role in repairing DNA damage, scientists have not fully understood its specific mechanism. In the study of PARP function, scientists also discovered a strange phenomenon: PARP-1 knockout mice develop normally, and cells isolated from this mouse show increased levels of sister chromatid exchange [9 , 10]. Sister chromatid exchange is thought to be the result of DNA recombination or recombinant repair. This means that in addition to PARP-1, there are other ways to repair DNA damage. Later, British scientist Thomas Helleday found that when PARP-1 was inhibited, DNA damage could be repaired by homologous recombination [11]. Homologous recombination is one of the ways to repair DNA double-strand breaks. There are many proteins involved in this repair method, such as BRCA, ATM, RAD51, etc., among which BRCA protein is the most well known. The story is here, and the "constructive death" great speculation for cancer treatment is about to become a reality! Convergence of God At this time, it is still the British who promote the story. On April 14, 2005, two research teams in the UK published important research results in the same issue of Nature [12,13], which confirmed for the first time that there was a “synthesis death†between PARP inhibitors and BRCA1 or BRCA2 mutations. "Interaction. It turns out that in cells with normal BRCA function, when PARP is inhibited, single-strand breaks in cells continue to increase, and once a single-strand break encounters a replication fork, DNA damage becomes a double-strand break. At this time, the homologous recombination involved in BRCA will take on the responsibility of repairing double-strand breaks and ensure DNA integrity [14]. However, in the BRCA-mutated cancer cells, the path of homologous recombination is no longer feasible, and the function of PARP is inhibited. In the face of a large number of double-strand breaks, cancer cells can only choose one. A fast but very error-prone method of DNA double-strand repair, and once it fails, the cancer cells will "snap". This combination is amazing! Therefore, with the wonderful concept of "synthesis to death", a large number of PARP inhibitors went to clinical trials. Restless At this time, many familiar figures have been seen in the clinical trials of PARP inhibitors. For example, AZD2281, which is later Olapari. The research team began recruiting solid tumor patients for phase I clinical trials in June 2005 [15], and published two papers [12,13] in Nature for only two months. The development of PARP inhibitors is evident. In this craze, there is also a "puppet" PARP inhibitor - iniparib. At the 2009 ASCO meeting, iniparib was amazed by the fact that infusion therapy with gemcitabine and carboplatin alone, combined with gemcitabine and carboplatin, resulted in a median progression-free survival (PFS) in patients with metastatic triple-negative breast cancer. Extending from 3.6 months to 5.9 months, the overall survival was extended from 7.7 months to 12.3 months [16]! Such a beautiful phase II clinical data, how can we not let the industry boil! However, the good times are not long. In January 2011, just one week after the results of this phase II clinical complete study published in the New England Journal of Medicine [16], the relevant drug companies announced that the phase III clinical trial of iniparib failed [17]. Not long after, a phase II clinical trial of olapari for ovarian cancer was also declared: compared to placebo, although olaapali extended the patient's PFS by 3.6 months (8.4 months vs. 4.8) Months), but does not extend the overall survival of patients [18]. Successive failures are like a cold water under the clear sky, and the enthusiasm of the PARP inhibitor development market is instantly ruined. Many pharmaceutical companies have lost confidence in PARP inhibitors, and even pharmaceutical companies have begun to resell their products. Of course, not everyone has lost their enthusiasm for PARP inhibitors. In 2012, Anand Patel and Scott H of the Mayo Clinic. Kaufmann et al. confirmed that iniparib is not a true PARP inhibitor and does not guide the research work of PARP inhibitors [19], which has revived the PARP inhibitor development market. The study allowed Olapali's research team to reanalyze the data from the Phase II clinical trials [18] mentioned above, and they were pleasantly surprised to find that in the group of patients with BRCA mutations, Ola compared to placebo. Parry's PFS was extended by 6.9 months (11.2 months vs. 4.3 months) [20], which is nearly twice the entire patient group (3.6 months). Encouraged by this, the Phase III clinical trial of olapali monotherapy maintenance therapy for patients with ovarian cancer carrying the BRCA1/2 mutation was initiated again in 2013. The final results showed that patients with platinum-sensitive recurrent ovarian cancer carrying the BRCA1/2 mutation were treated with olaipril compared with placebo and median progression-free survival (PFS) was extended to 19.1 months ( In the placebo group (5.5 months), the risk of disease progression or death was reduced by 70% [21]. In 2014, Olapali was approved by the European Food and Drug Administration EMA and the US FDA for the treatment of ovarian cancer with BRCA1/2 mutation. On this difficult and tortuous drug development road, there is a PARP inhibitor that is a rising star, but it can be step by step and post-production. Swing up In 2009, when both rucaparib and olapari had entered clinical research, a compound code-named MK-4827 was first published in academic journals[22] to meet the world. Later, Niraparib. Drug developer Philip Jones et al. found that MK-4827 inhibited the proliferation of BRCA-1 and BRCA-2 mutant cancer cells in the 10-100 nM concentration range; in the BRCA-1 deficient tumor transplantation model, MK-4827 was used alone. A good anticancer effect can be obtained and the drug is well tolerated. More importantly, in vitro, the inhibitory activities of MK-4827 on PARP-1, PARP-2 and PARP-3 were 3.8 nM, 2.1 nM and 1300 nM, respectively, ie MK-4827 versus PARP-1 and PARP-2 has a higher selectivity, which is the key to why Nilapali can be later. Prior to the official entry into clinical studies, Nilapali's R&D team used a number of preclinical models to validate, not only with different cell line drug validation studies, but also with various animal models [23]. In conclusion, it was ensured that nilapali can achieve its pharmacodynamic goals of PARP inhibition by once-daily oral administration. After everything was done, Nilapali entered the clinical trials step by step. In 2013, a paper in The Lancet Oncology [24] published the results of Phase I clinical trials of nilapali, involving 100 patients with advanced solid tumors (some patients carrying BRCA1 or BRCA2 mutations) doses. The incremental test gives the recommended dose for subsequent clinical trials and it is recommended to further evaluate the therapeutic effect of nilapali in patients with homologous recombination defects. The results of the next clinical trials can be said to live up to expectations, even unexpected. On December 1, 2016, Nilapali's Phase III clinical trial of platinum-sensitive recurrent ovarian cancer was unveiled. The results of this heavy clinical study were published in the New England Journal of Medicine [25]. Compared with placebo, nilapali extended the PFS of patients carrying the BRAC germ line (gBRCA) mutation from 5.5 months to 21.0 months. This result is really cheering! Even better, even patients without BRCA mutations can benefit from treatment with nilapali. Compared with placebo, nilapali extended the PFS of patients who did not carry the gBRCA mutation from 3.9 months to 9.3 months; among them, the PFS of patients with homologous recombination-positive patients was extended from 3.8 months to 12.9 months. This means that the use of nilapali does not require consideration of BRCA mutations. With such good results, Nilapali was quickly approved by the US FDA in March 2017 for recurrent epithelial ovarian cancer in adults with complete or partial remission (complete or partial response) after platinum-based chemotherapy. Maintenance therapy for fallopian tubes or primary peritoneal cancer [26]. Although Nyala Palibi Olapali and Recapalli, which are listed a few years later, this does not prevent Nila Pali from becoming a shining star because it is the first defect that does not require consideration of BRCA mutations or homologous recombination. The state is available for the treatment of PARP inhibitors. Subsequently, Olapali and Recapalli were approved by the US FDA in August 2017 [27] and April 2018 [28], respectively, and obtained the same indications. Future Half a century has passed, from the first discovery of PARP to the approval of several PARP inhibitors for ovarian cancer treatment, the surprises of PARP inhibitors continue. In October 2018, Nilapali was approved for marketing in Hong Kong, China [29] for the maintenance of all patients with platinum-sensitive recurrent ovarian cancer. In combination therapy with other cancers, such as triple-negative breast cancer, a phase II clinical trial showed that the objective response rate of nilapali combined with PD-1 monoclonal antibody (pembrolizumab) reached 29%, higher than pembrolizumab alone. 5–18% objective response rate, patients benefit significantly [30]; In addition, clinical trials for non-small cell lung cancer, prostate cancer, PARP inhibitors combined with chemotherapy or radiotherapy are underway [31]. In addition to cancer, PARP inhibitors have shown great potential in preclinical studies of non-neoplastic diseases such as myocardial infarction, stroke, chronic neuritis, etc. [32]. I believe that future PARP inhibitors will bring more good news. JRT usb interface distance sensor board adds a USB port to a Laser Distance Sensor, inexpensive laser distance measurement sensors, allowing customers directly using GBeacon-2.3-Fx.exe software to control the rangefinder sensor. Usb laser distance sensors are the the most popular model devices, it works by measuring the phase of an visible-red laser beam that reflects on the target. Usually, customers would like to choose CH341SER.EXE version to test. USB Laser Distance Sensor,Laser Range Finding,laser tool measurement,distance measurement device Chengdu JRT Meter Technology Co., Ltd , http://www.rangesensors.com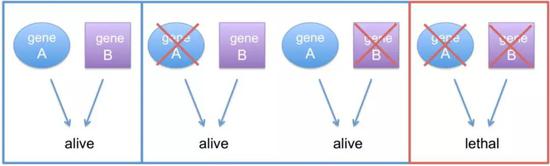
"Synthetic lethal" schematic [1] 
(Source: pixabay.com) 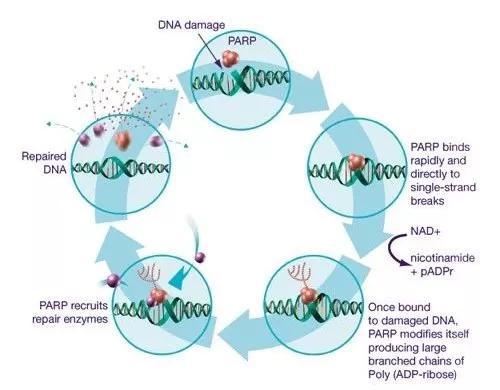
(Source: libbyshope.com) 
(Source: pixabay.com) 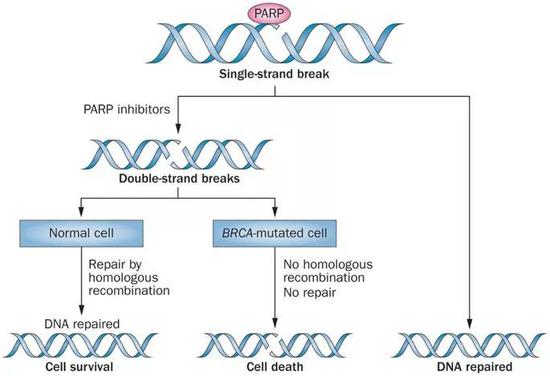
Schematic diagram of "synthesis and lethality" of PARP inhibitors and BRCA [33] 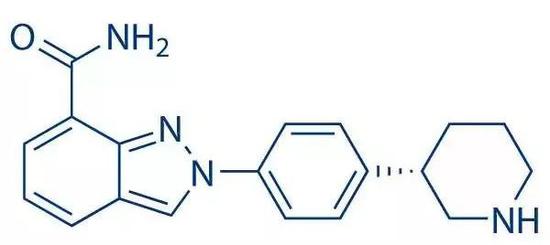
Nilapali structure 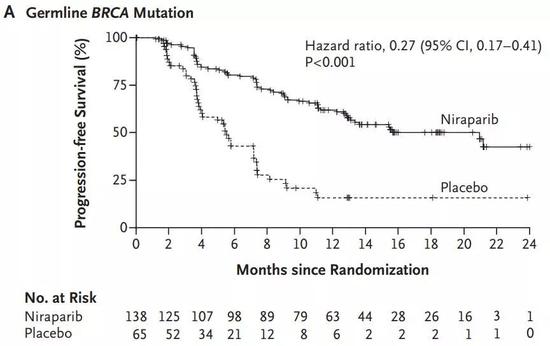
Nilapali allows prolonged PFS in patients with gBRCA mutations [25] 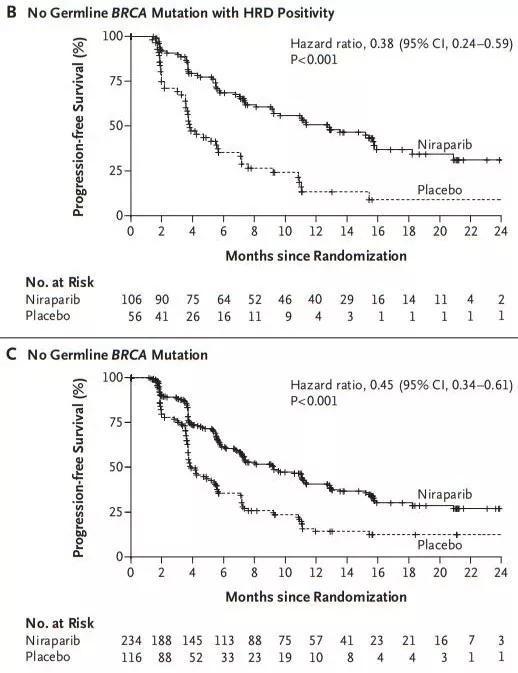
Patients with non-BRCA mutations can also benefit [25] 