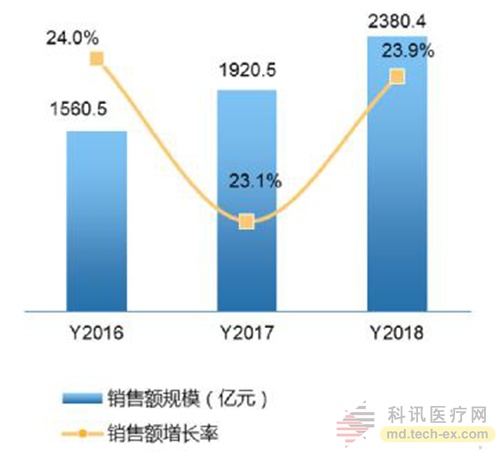
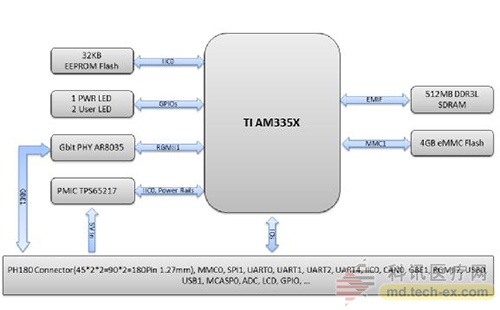
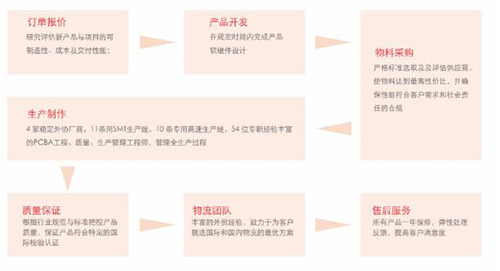
Release date: 2017-02-21 With the continuous development of medical informationization, mobility, and intelligence, residents' attention to their own health has continued to strengthen, and the global medical electronics industry has continued to maintain rapid growth in recent years. At the same time, the huge population base and the rapidly growing aging population have brought about a growing demand for medical services. In addition, the national health care is closely related to the national strategy. It has always been the focus of the government. In recent years, China has become an important part of the world. One of the medical electronics markets. According to the "2016-2021 China Portable Medical Electronics Industry Market Demand and Investment Advisory Report" data, China's medical electronics market is expected to continue to grow in 2016-2018, with an average compound annual growth rate of 23.7%, 2018 sales. It will climb to 238.04 billion yuan. Figure 1. China's medical electronics market size and growth forecast data from 2016 to 2018 The accuracy of local medical electronic diagnosis is constantly improving, and the trend of integration and intelligent research and development is highlighted. "The scale of China's medical electronics industry has maintained steady and rapid growth in recent years. While products are constantly pursuing cost reduction, the most important concern is the humanization requirements of safety, reliability, ease of use, etc." The local medical electronics are presenting two major research and development trends and development directions: First, the diagnostic accuracy is not the highest, only higher, because the diagnostic accuracy will directly affect the equipment diagnosis results, which is bound to be the focus of future product design, second , showing the trend of “integrated and intelligentâ€, such as monitoring equipment integrating diagnosis, monitoring, image, communication and other functions.†Selected by many medical and electronic customers, the company's core board verification "integrated, intelligent" research and development trend Back to the core, the advanced development of medical equipment products is inseparable from the continuous innovation of medical electronics, and the “big burden†of medical electronics in the field has become increasingly apparent. The core board Mini8600B is the solution developed by Inventec for medical electronics. It was selected by nearly 20 medical electronic equipment companies and became the IVD for the development of blood analyzers, nephrology (urine) monitors, myocardial detectors, maternal and child care, etc. (In vitro diagnostics) The core control board of the instrument. The Mini8600B is an ARM core board based on the TI Cortex-A8 AM3358 processor and is compatible with the AM335x series of processors. The core board is small in size, with 512MByte DDR3 SDRAM and 512MByte NandFlash onboard. The two internal interfaces of the AM335x processor are led out through two 2*40pin board-to-board connectors, which makes it easier for customers to design the product function board or interface board more quickly. In addition, the core board Mini8600B supports Linux3.2.0, WinCE 7 and Android4.0 operating systems. Infineon provides developers with a complete software development environment and comprehensive technical support to help customers reduce product development cycle and realize products. Quick time to market. Figure 2. Front and back views of the Infinet Mini8600B core board The SOM-PH8700 core board is an upgraded product based on the Mini8600B. It is also based on the TI AM3358 (AM335x) series of chips. The processor integrates the ARM CortexTM-A8 core up to 1GHz, and the storage uses 4GB eMMC to provide more system. Reliable data storage, larger data storage space. The core board SOM-PH8700 is most worth mentioning. Its hardware interface adopts the PH180 interface standard defined by Infinex. It is compact and has a wide range of peripheral interfaces. It includes two 90Pin 1.27mm pitch IO expansion interfaces, for example, 3 x I2C, 2 x SPI, 2 x CAN, 6 x UART, 3 x MMC, 2 x I2S, 1 x LCD, 1 x RGMII, GPIO, 1 x Gigabit Ethernet, power supply, etc. Rich peripheral interface makes SOM-PH8700 connected The backplane can be adapted to a variety of medical devices, while the standardized design also makes subsequent product updates easier. Figure 3. Block diagram of the SOM-PH8700 system In fact, the core board Mini8600B and SOM-PH8700 are not limited to medical electronics, and both play an important role in many fields. The core board Mini8600B can be used in portable navigation systems, digital video set-top boxes, portable educational/game devices, industrial automation, building automation, human machine interfaces, teaching/medical equipment and more. The SOM-PH8700 will be used in a wide range of applications, including gaming peripherals, home and industrial automation, consumer medical devices, printers, smart toll collection systems, smart vending machines, weighing systems, educational terminals, and advanced toys. Different needs in various fields. International operations + quality control, continue to introduce the source of high-performance embedded solutions As we all know, medical electronic solutions focus on high performance and high quality requirements, and Infineon is able to continue to introduce cost-leading, high-performance embedded solutions, thanks to the introduction of professional quality management experts and operational personnel in the early stage, reorganizing and constructing The company's business processes have brought production and operation integration and supply chain management to an unprecedented level. It is reported that Infinex has successfully passed the ISO9001:2008 system certification in May 2015. Figure 4. Infineon's operational management process highlights Instructor R&D Director Zhang Jianwei emphasized: “The next generation of innovation requires the latest solutions and has become the industry consensus. Internationalized operation team, scientific system project management experience, reliable production line, quality control ability throughout the product life cycle. , to ensure the quality of the British special products, but also to ensure that the British special can undertake and timely delivery of large quantities and retail orders at home and abroad, to help customers purchase more suitable products, greatly reducing the overall development difficulty and speeding up the development process." Source: Electronic Products World Brand computer chips, dual-frequency integrated circuits, cross-magnetic induction detection, bringing higher accuracy, reliability and stability. 2. Automatic control system, using special magnetic materials and unique technology, the detection accuracy of the probe is particularly high, and the accurate position of the broken needle is displayed in 8 detection areas. 3. Dual probe design, double detection function, higher safety performance, compared with other types of needle detectors, at the same detection height, the sensitivity is increased by 10%. 4. Automatic detection, automatic induction, automatic stop when no detection is needed, 0-10 sensitivity adjustment, when there is a broken needle, the conveyor belt automatically stops and returns backwards. 5. With a counting function, it can count the number of qualified or unqualified products. 6. It has two alarm modes: sound effect and light alarm. 7. Luxurious appearance, novel style, time-saving and power-saving, simple operation Rapid PCR detection,Rapid Detection Rapid Detection PCR Kit Viral Antigen Detection Jiangsu iiLO Biotechnology Co., Ltd. , https://www.sjiilobiotech.com