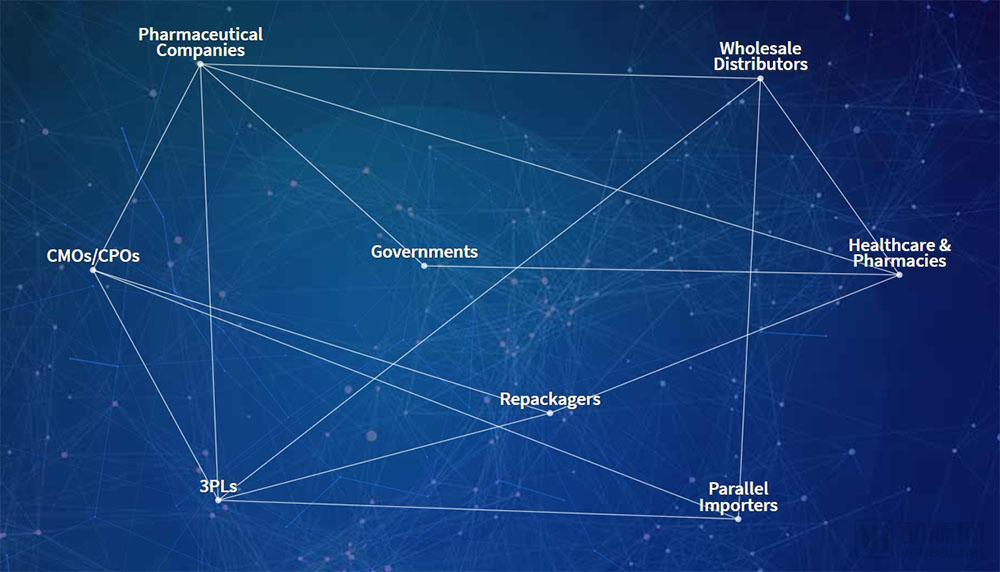
At this stage, the problem of counterfeit drugs in the pharmaceutical market cannot be underestimated. The World Health Organization released a report in 2015 that, on a global scale, one-tenth of the drugs circulating in the market are counterfeit drugs, causing hundreds of thousands of deaths each year, resulting in nearly $75 billion in industry losses. In 2013, after the US Drug Supply Chain Security Act was enacted, pharmaceutical companies throughout the supply chain were required to better track drugs and record the entire process from the manufacturer to the patient. The bill stipulates that the unit-level drug can be prosecuted for 10 years, while providing the industry with investment opportunities to achieve the services and equipment needed for compliance. Counterfeit drug monitoring and drug tracking have become the entry point for TraceLink, which currently serves more than 450 life sciences companies worldwide. What does TraceLink do mainly? Founded in 2009, TraceLink's core business is to block the trading channels of counterfeit drugs through the drug tracking SaaS platform, trying to find out all the fake prescription drugs. According to Arterial, the company's Life Sciences Cloud software helps manufacturers, distributors, and pharmacists track drugs in the supply chain and comply with country-specific tracking requirements in their supply chains. TraceLink provides a unique serial number identifier for each drug, and the serial number identifies and verifies the suspect drug and reacts to the integrated system. In addition, the use of date, inventory, logistics and other information can also be used to understand the circulation of drugs, thus providing decision support for pharmaceutical manufacturers. At present, TraceLink's tracking network has covered nearly 300,000 partners, including the US and EU markets. For customers such as hospitals and pharmacies, TraceLink's drug management software supports interactive features. The dispensing pharmacist or doctor can communicate specific information about the drug with the drug manufacturer to optimize the treatment process. The serial number of the drug sold is linked to the patient information, and when the drug has a problem, it is also convenient to recall or track the patient information. In June 2018, TraceLink announced a $60 million Series D round of financing, with 18 investors in this round, including Goldman Sachs Group, FirstMark Capital, Volition Capital and F-Prime Capital, which previously led their C-round financing. After the current round, TraceLink raised a total of $140 million in financing and is preparing for an IPO. Not long ago, the company also announced its quarterly revenue and customer growth rate. In 2018, the company's revenue in the first quarter increased by 69% compared with the same period of last year. Among them, Asia-Pacific revenue increased by 386%, EMEA revenue increased by 270%, and Indian revenue increased by 159%. In May 2018, TraceLink signed a strategic cooperation agreement with Beijing Atron Technology Co., Ltd., and the two parties agreed to carry out in-depth cooperation in the field of pharmaceutical digital supply chain and global compliance management in Greater Asia and Greater China. They plan to build a strong level 1 to Level 5 full-scale, one-stop drug safety and digital supply chain solution to provide qualified Chinese pharmaceutical companies with advanced services in international industry norms and standards. Shabbir Dahod, president and CEO of TraceLink, said: “Thousands of companies in the pharmaceutical supply chain need to know how to sell drugs under time-bound regulations. TraceLink wants to provide these companies with both regulatory and revenue-generating solutions. Program to help them safely deliver the drug to the patient." Based on technology and experience, develop a “one-stop†service in the supply chain Shabbir Dahod, President and CEO of TraceLink “At TraceLink, we've combined decades of innovative technology with supply chain business processes to build cloud-based web applications,†Dahod said. “We firmly believe that this will greatly improve supply chain management and operations in the life sciences industry.†Shabbir Dahod is President and CEO of TraceLink and a co-founder and board member of the company. In the early days of the founding of the company, he hoped to establish a company for life sciences companies that uses science and technology to optimize their business management. Working with global trading partners, the company plans to improve the global pharmaceutical supply chain, including pharmaceutical, packaging, distribution and dispensing. For more than a decade, Dahod has been committed to researching and implementing the integrity of the pharmaceutical supply chain to protect the interests of patients around the world. As a long-term leader, Dahod has long recognized the important role that product traceability technology plays in improving the life sciences supply chain and the enormous impact it has on saving lives. In the early 21st century, Dahod learned during his collaboration with the Massachusetts Institute of Technology (MIT) Auto-ID Lab that counterfeit drugs were being prematurely prevalent throughout the United States, posing a serious life threat to unsuspecting patients. Since then, he has been immersed in the Internet of Things (IoT) related research, plans to promote the entire process of medical product transportation to the digital cloud. In May 2003, he founded SupplyScape and was a leader in the computer market and multimedia creation technology. Dahod said that nearly 100 companies had used SupplyScape's solution, which focused on addressing product safety and regulatory challenges due to lack of supply chain visibility. By applying the innovative technology of SupplyScape, the drug footprint is managed and presented in digital form, ensuring that drugs are safely delivered from the manufacturer to the patient, completing all steps in between. A few years before DSCSA became law, Dahod introduced TraceLink, introducing the concept of a life science cloud. This is a cloud-based software hosted by Amazon Web Server, which has become the world's largest traceable supply chain system. In addition, Dahod worked with Microsoft co-founder Paul Allen at Asymetrix early in his career. Paul Allen, a US business giant, investor and philanthropist, co-founded Microsoft with Bill Gates. In February 2018, he was the 43rd richest man in the world with an estimated net worth of $25 billion. During his time with Allen, Dahod developed a number of emerging Web product technologies, including early plans for e-commerce, natural language queries, online sports, digital media and Java development tools. After that, Dahod served as a senior executive at Allen Group and became a senior executive after joining Microsoft. Dahod advocates the use of emerging XML standards and Internet communication tools to lead teams in knowledge management related learning. Why use TraceLink's Life Sciences Cloud? As a company that provides cloud software for the life sciences, TraceLink not only provides drug tracking services to businesses, but also encourages them to exchange data with partners. Dahod said that every company that joins the TraceLink network can connect with dozens or even hundreds of companies through their cloud software. This not only speeds up the efficiency of cooperation between companies, but also greatly saves costs. Regarding the concept of cloud, Dahod said: "We can start 100 servers to perform tasks, and once the task is completed, these 100 servers can disappear. This is a powerful function that can be used globally to solve computers. The problem of limited resources." Based on the use of TraceLink cloud software, Dahod emphasizes that the amount of data storage contained in a virtual solution is huge. Cloud storage is great for supply chain applications. It not only stores trillions of projects in the supply chain, but also processes them in real time, increasing flexibility and meeting company requirements. The benefits of serializing data Serialization opens up new areas of data complexity, and now drug products in the supply chain must be presented in data, with targeted delivery at the right time, route, and location. TraceLink's cloud software reduces the labor and cost of establishing peer-to-peer work connections. In addition, serialization involves many different data formats, and the absence of standard formats increases the complexity of product traceability and data exchange. TraceLink's cloud software ensures that data is sent to partners in the most convenient and direct format, often in the format EPCIS (Electronic Product Code Information Service). EPCIS was first used in electronic tags and is used as a standard for IoT and automated data collection. In September 2015, EPCIS officially became the ISO standard. It is defined as a commercial data standard for the collection and sharing of visual data information between different applications. It realizes the full visualization of physical objects and virtual objects in business processes: This includes use in trade projects, recyclable assets, pallets, electronic music downloads, e-books, coupons, and more. Flexible exchange of data information TraceLink Cloud Software's unique network architecture enables rapid configuration of trade discovery and data exchange. Since October 2014, TraceLink's network has included more than 270,000 supply chain endpoints, and according to the Arterial Network (WeChat: vcbeat), they have saved the industry billions of dollars in point-to-point integration costs. In addition, the company is committed to providing the following services and support: - Instantly and unrestrictedly communicate with more than 270,000 trading partners through a single data connection to TraceLink Cloud Software. - Enter the global trade market in a timely manner by quickly understanding and adopting new national policies. - Simplify data integration through partner's preferred file format, file size, naming management and transfer order to efficiently exchange information. - Rapid deployment of new software that keeps pace with the company's business, based on the needs and development of trading partners, for large-scale serialization and transaction processing of data. - Out-of-the-box synthesis with third-party systems (ERP, WMS, LMS, edge and government systems) to simplify configuration and properly load map information about the provider. - Automated data replication across multiple data centers with a unique cloud architecture that maximizes the computational capacity required for configuration. - Gain supply chain operational information for real-time visibility. Drug recalls, drug verification, and product direction can be completed if necessary. Automated verification that meets GxP compliance requirements GxP refers to the different stages and regulatory relationships of drug assurance throughout the life cycle. It covers GLP/GRP (preclinical stages: chemistry, pharmacy, toxicology), GCP (clinical stage I, II and III), GMP/GCP (generating/IV clinical), GMP (commercial production), GSP (dealer), GUP (hospital/consumer) and other sectors. Traditionally, verification typically involves a manual process that is managed by weeks and expertise. This is a major challenge for large and small companies in the industry that are constrained by new regulations and developmental conditions. TraceLink provides solutions that keep the company's systems up-to-date and in line with industry best practice GxP compliance standards. In this case, companies and their trading partners can run and use the same version of communications software to increase productivity. Comparison of TraceLink and the Li Kang Pharmacopoeia The Likang Pharmacopoeia contains a total of 6500 kinds of Chinese and Western medicines and Chinese medicine prescriptions. It can be classified according to subjects, and can quickly locate drugs and retrieve them in full text. At the same time, it can query commonly used drugs, doses and side effects, and has fewer types. It is a popular non-professional drug manual and is an essential electronic reading for medical workers. Compared to TraceLink, the Likang Pharmacopoeia is mostly used for drug enquiries. The drug information is limited, and the version needs to be updated independently. The software can be downloaded and installed before use. Manual Massage Collapsible Foot Spa Massager The Manual Massage Foldable Foot Spa Massager is a portable compact device designed to give your feet a relaxing massage experience. Its foldable design makes it easy to store and transport, making it ideal for the frequent traveler.
Overall, the Manual Massage Foldable Foot Spa Massager is an excellent choice for anyone looking to relieve foot pain and tension in the comfort of their own home.
Manual Massage Collapsible Foot Spa Massager,Foot Bath Massager Machine,Electric Heated Foot Spa,Foot Bath Tub Huaian Mimir Electric Appliance Co., LTD , https://www.mmfootbath.com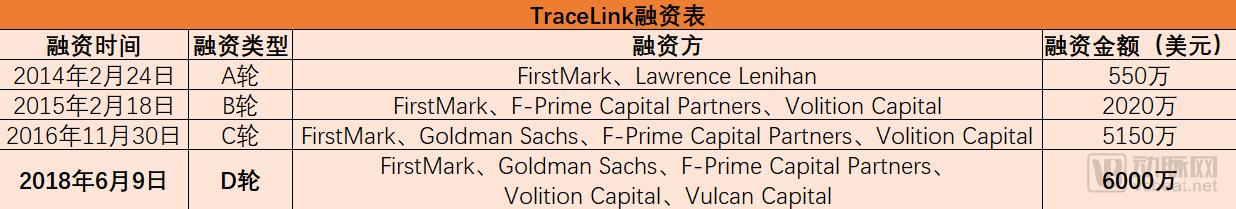

Massagers typically have multiple massage rollers that apply pressure to different areas of the foot, providing a deep-tissue massage that can help relieve tension and improve circulation. Some models also feature heating and vibration to further enhance the massage experience.
To use the massager, you simply fill it with water and turn it on. The water will heat up and the massage rollers will go into action to provide a soothing massage that will help relax your feet.