Vault Room Construction,Vault Room In House,Vault Room Door,Vault Room Hebei Yingbo Safe Boxes Co.,Ltd , https://www.yingbosafes.com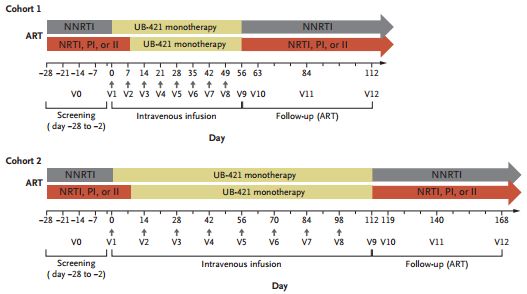
Significant progress! New antibodies can suppress HIV for up to 4 months
Medical Network April 22, according to a recent online study published in the New England Journal of Medicine, for patients who are experiencing a short-term suspension of antiretroviral therapy (ART), a regular injection of one can Antibodies that block the binding site of HIV at human immune cells can suppress HIV levels for up to four months. The results of Phase 2 open studies indicate that this antibody, called UB-421, is safe and does not induce HIV-resistant antibodies.
Image source: New England Journal of Medicine
The study was partially supported by the National Institute of Allergy and Infectious Diseases (NIAID) and United Biopharma, Inc. The study was conducted in Taiwan and was led by Dr. Chang Yi Wang, Chief Scientific Officer and Chairman of United Biopharma, Inc.
Twenty-nine HIV-controlled volunteers stopped their daily oral antiretroviral treatment regimen after the first injection or a week later. Fourteen study participants injected UB-421 eight times a week, and 15 participants injected high-dose UB-421 eight times a week. At the end of the 8 or 16 week treatment period, all volunteers resumed their previous ART regimen and were evaluated at 8 weeks follow-up. Except for one participant who discontinued the study due to a mild rash, the two groups maintained a good state of HIV suppression during the entire treatment period without antiretroviral therapy (plasma HIV RNA levels below 20 copies/ml) ).
In previous experiments, extensive neutralizing antibodies (bNAbs) that injected proteins that target the virus itself inhibited HIV for about two weeks, but the rapid mutation of HIV led to antibody-resistant strains that ultimately rendered the treatment ineffective. In theory, UB-421 avoids this possibility by blocking the stable human proteins that HIV uses to infect T cells. In fact, no resistance to UB-421 was found in this study.
Since this small study did not include a comparison group receiving placebo injections, Taiwan and Thailand plan to conduct further studies to assess the safety and efficacy of UB-421 as an HIV treatment. In a related study, NIAID researchers are currently evaluating the safety of regular injections of two potent bNAbs that prevent the development of resistant HIV strains by targeting two distinct regions of the virus.
Reference materials:
C Wang et al. Anti-CD4 Antibody UB-421 on HIV-1 Rebound after Treatment Interruption, New England Journal of Medicine (2019). DOI: 10.1056/NEJMoa1802264