On March 21, Shenzhen Market and Quality Supervision and Management Committee (Baoan Bureau) held the 2017 Medical Device Manufacturing Enterprise Supervision Conference, Deputy Director of Haiping Bureau of Hai'an Bureau, Medical Devices, Shenzhen Medical Device Industry Association, Special Inspectors and Over Representatives from 150 medical device manufacturers attended the meeting. The supervision department of Baoan City inspected the denture production enterprise. The reporter learned from the meeting that the Baoan Bureau will conduct a joint inspection of the production quality management system of medical device manufacturing enterprises from April to July, and conduct on-site inspections of 35 enterprises in the jurisdiction. If it is found that the suspected violation of laws and regulations will be seriously investigated and dealt with. Recently, the staff of Baoan Bureau first went to some denture enterprises to carry out the inspection. In the Shenzhen Zhilibao Dental Preparation Co., Ltd., located in Xixiang Street, Baoan District, the law enforcement personnel of Baoan Bureau carefully inspected the production management specification documents and production of the denture enterprise. Site and production records. Huang Zengfeng, general manager of Zhili Dental Preparation Co., Ltd., said that Xixiang is one of the gathering places for denture manufacturers, and recently established the Baoan Denture Professional Committee. "We are supporting the inspection of medical enterprises by the government departments, because this will allow the entire industry to develop better." Huang Zengfeng said. It is reported that there are 210 medical device manufacturers in the Bao'an Bureau, accounting for 30.6% of the city, including 23 of the three types of enterprises, 112 of the second category, and 75 of the first class, which are included in the national key and provincial key supervision enterprises. Family. The meeting conveyed the work requirements of the National, Provincial and Municipal Bureau of Medical Device Supervision Conference in 2017, and proposed to actively promote quality management practices, implement classified classification and supervision, and strengthen matters in accordance with the requirements of “four strictest†and “four have two responsibilitiesâ€. In the post-event supervision, we will strictly guard the source, strictly control the process, strictly control the risks, and strive to protect the work safety of the public. We have sorted out the six key tasks of the medical device supervision in 2017 and the six major inspection points, and explained in detail. "Combined Inspection Work Plan for Production Quality System of Medical Device Manufacturing Enterprises in Baoan District" and "Administrative Measures for Classification and Classification of Medical Device Manufacturing Enterprises in Baoan District".
Shanghai Rocatti Biotechnology Co., Ltd. is a professional enterprise of rehabilitation and medical device products with sales and service as the main body. Since its establishment, the company has been committed to the development of medical devices. Its products involve the production of epidemic prevention materials such as gynecology (obstetrics), Urology, neurosurgery, kn95 medical protective masks, daily use flat masks, and the research and development of total nutrition slimming food. It can provide a complete range of first, second and third class medical device configuration schemes for general hospitals and specialized hospitals.
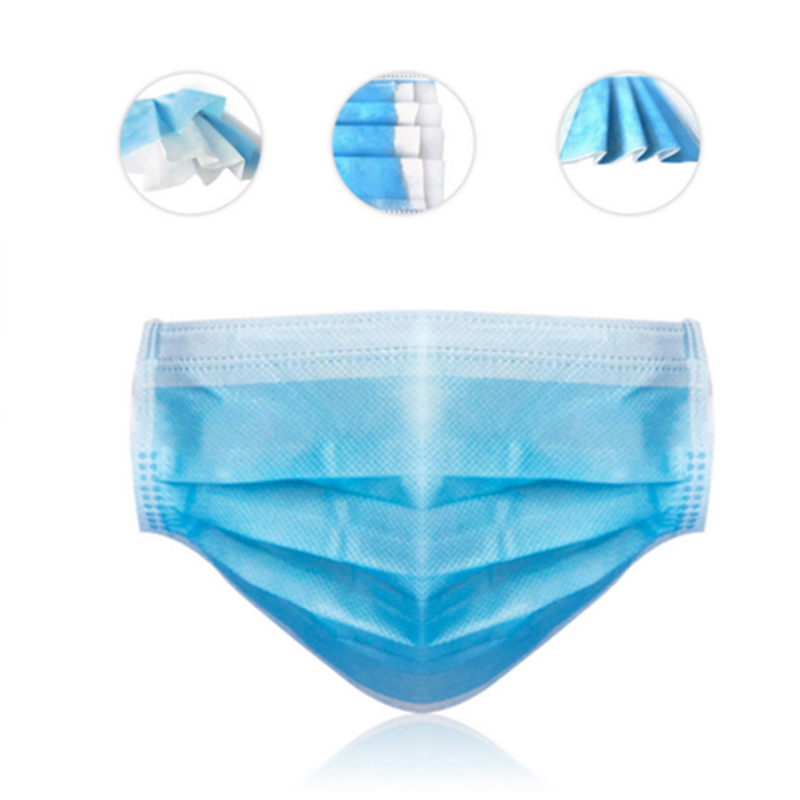
Nonwoven Face Mask,Non-Woven Facial Face Mask,Non-Woven Face Mask Ffp2,Non-Woven Surgical Face Mask Shanghai Rocatti Biotechnology Co.,Ltd , https://www.shljdmedical.com