Canned Tuna,Fresh Canned Tuna,Frozen Canned Tuna,Canned Skipjack Chunk ZHEJIANG RETRONX FOODSTUFF INDUSTRY CO.,LTD , https://www.retronxfoods.com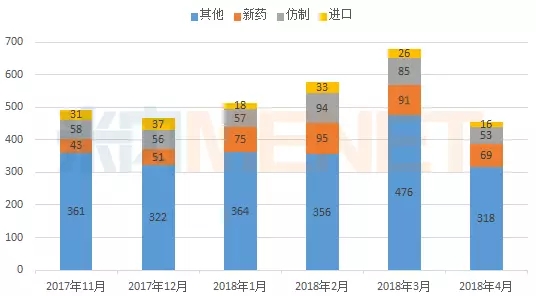


Three injections reported consistency evaluation of the first authorized generic drug in China to enter CDE
Medical Network May 4th
Summary
Evaluation of the consistency of three generic injections for generic drugs
Application of Karelizumab for injection of Hengrui Pharmaceutical PD-1 monoclonal antibody
There are two more CAR-T drugs for clinical application.
AstraZeneca and Merlot Pharmaceuticals jointly filed the first domestically licensed generic rosuvastatin calcium tablets
Merck's nine-valent human papillomavirus vaccine (Saccharomyces cerevisiae) was given conditional approval for listing
The State Food and Drug Administration announced the third batch of catalogues for the evaluation of generics through generics, involving 7 varieties.
Overall undertaking
According to MED China Drug Evaluation Database 2.0 statistics, in April 2018, CDE hosted 456 drug registration applications, a year-on-year decline.
Figure 1: Application for registration of drugs by CDE from November 2017 to April 1818 (according to the acceptance number)
(Source: MED China Drug Evaluation Database 2.0, the same below)
Coordination evaluation application status
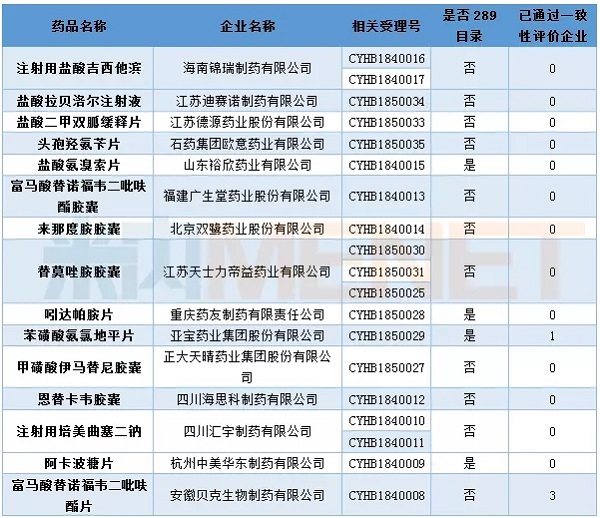
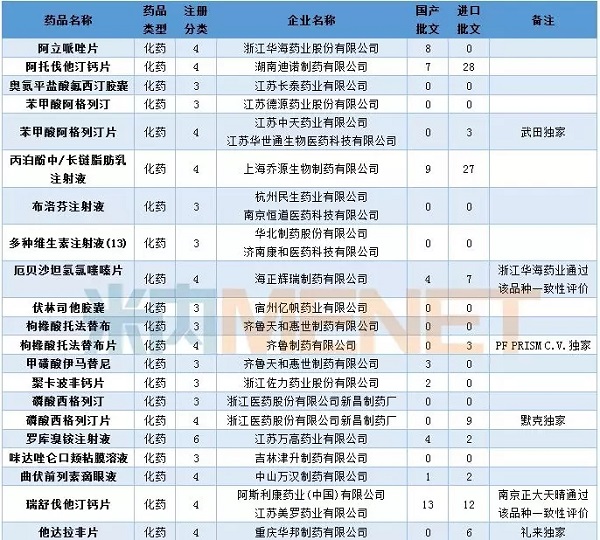
In April, 19 consecutive evaluation applications entered the CDE, involving 15 varieties, three of which were injections, namely, gemcitabine hydrochloride for injection of Hainan Jinrui Pharmaceutical, and labetalol hydrochloride for Jiangsu Desino Pharmaceuticals. Sichuan Huiyu Pharmaceutical's injection of pemetrexed disodium.
At present, one company of amlodipine besylate has passed the consistency evaluation, while three companies of tenofovir disoproxil fumarate have passed the consistency evaluation.
   Table 1: Acceptance of generic drug conformity assessment in April 2018
Domestic first class new drug contractor
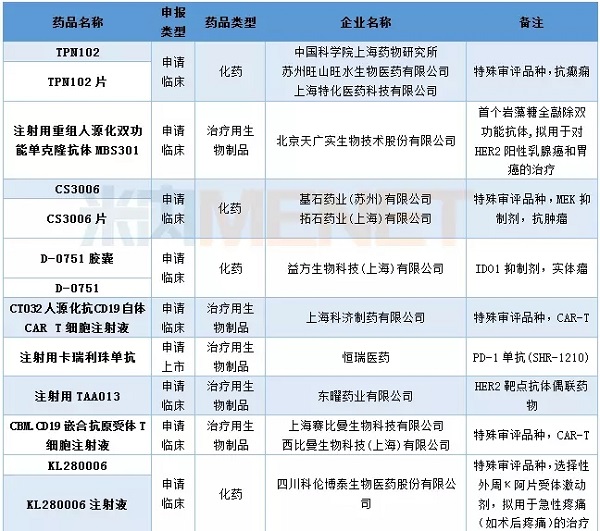
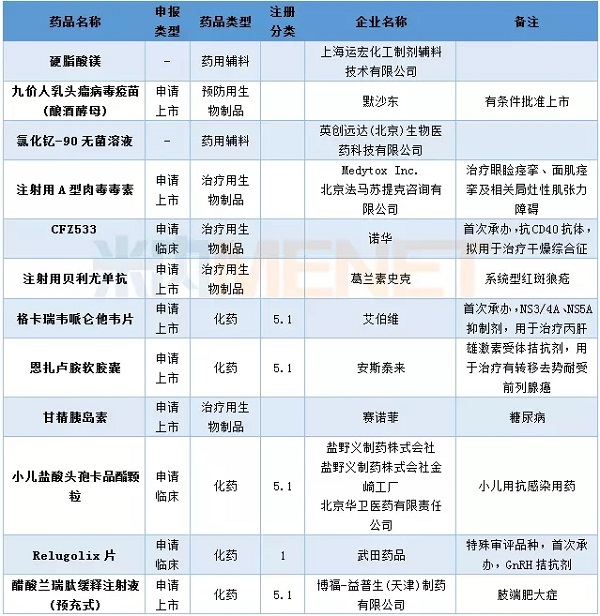
In April, CDE hosted 69 domestic new drug applications, of which 41 were for Class 1 new drugs, involving 22 varieties. Cinda Bio has resubmitted its PD-1 monoclonal antibody market application, Hengrui Pharmaceutical PD-1 monoclonal antibody (injection with carelibizumab) submitted a listing application, becoming the third domestic application for PD-1 monoclonal antibody listing Business . Shanghai Keji Pharmaceutical and Sibman Bio various CAR-T drugs enter the CDE, other conditions are shown in the table below.
   Table 2: Domestic Class 1 New Drugs in April 2018
Domestic generic drug contractor
In April, CDE has undertaken 53 generic drug applications, involving 33 varieties, of which 10 are currently domestic exclusive varieties. There are currently 46 domestic approvals and 4 import approvals for metformin hydrochloride sustained-release tablets, showing signs of homogenization. In addition, the application for the listing of rosuvastatin calcium tablets jointly submitted by AstraZeneca and Jiangsu Meiluo Pharmaceutical Co., Ltd. was initiated by CDE, which is the first authorized generic drug to be applied for listing in China. It is understood that the raw materials , packaging materials and production lines of this variety are consistent with AstraZeneca's “can be determined†original drug.
  Table 3: Imitation application for contract in April 2018
Import application
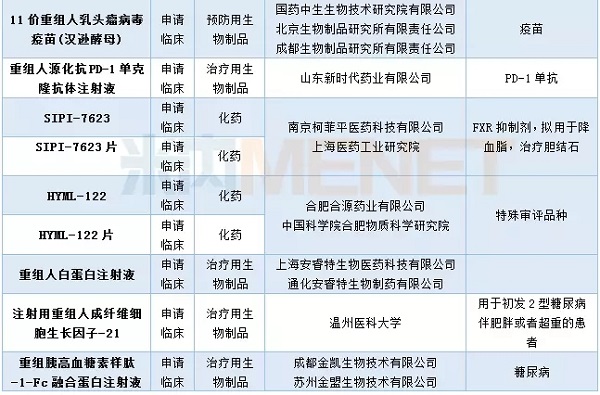
In April, CDE hosted 16 import applications involving 12 varieties. Novartis's CFZ533 and Aberdeen's gecareva pirenium tablets are among the first to be undertaken. Merck's nine-valent human papillomavirus vaccine (Saccharomyces cerevisiae) was submitted for approval in April and has been approved for approval.
  Table 4: The filing of CDE import applications in April 2018
Approved
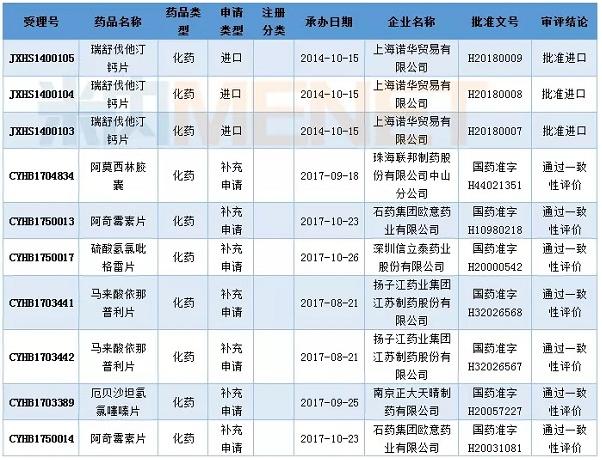
In April, the State Drug Administration issued the third batch of catalogues that have been approved to pass the evaluation of the quality and efficacy of generic drugs. This batch has seven varieties of specifications that pass the evaluation of the quality and efficacy of generic drugs.
   Table 5: Approval of partial applications in April 2018