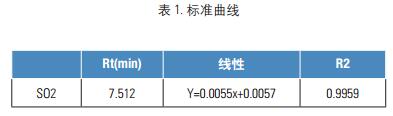
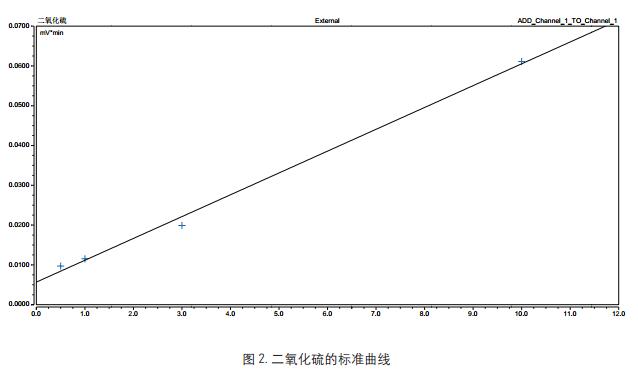
Key words Gas chromatograph; sulfur dioxide; TCD detector aims Recalling the detection of sulfur dioxide in the Chinese Pharmacopoeia of the 2015 edition of the Chinese Pharmacopoeia using the Thermo Scientific Gas Chromatograph (third method) introduction The use of sulphur fumigation is a common method used in the rough processing of some Chinese medicinal materials. The purpose is to prevent mildew, corrosion and dryness. There is currently no easy and effective alternative. Because sulfur dioxide is a strong reducing agent, it may affect the quality and efficacy of Chinese herbal medicines while causing changes in the active ingredients of traditional Chinese medicines. Furthermore, if the sulphur dioxide content in the medicinal material is too high, it may cause symptoms such as sore throat and stomach discomfort in the patient [1, 2] . In order to prevent the abuse or excessive use of sulfur fumigation in the rough processing of Chinese herbal medicines, and to ensure the quality, safety and effectiveness of traditional Chinese medicines, the National Pharmacopoeia Commission has established in 2003 the research on the determination methods and limits of sulfur dioxide residues in Chinese herbal medicines and decoction pieces. The method of measurement was started in the 2005 edition of the Supplement to the Chinese Pharmacopoeia. The guidance for the detection of sulfur dioxide is also given in the forthcoming 2015 edition of the Pharmacopoeia, and the gas chromatograph method is the first method for the detection of sulfur dioxide. This method is intended to provide a reference for users by using the Thermo Scientific GC to reproduce the 2015 edition of the Pharmacopoeia. instrument Trace1310 gas chromatograph with TCD detector (Thermo Scientific); Consumables Column: GS-GASPRO, 30 m, 0.32 mm Reagents and standards Solid paraffin: pathological grade pure grade: sodium sulfite, hydrochloric acid, mannitol, disodium edetate Experimental condition RSH three-in-one autosampler, headspace sampling method to balance temperature and time: 80 ° C, 10 min Preparation of control solution and test solution Preparation of the reference solution accurately weighed 500 mg of sodium sulfite reference substance, placed in a 10 ml volumetric flask, dissolved in a mixed solution containing 0.5% mannitol and 0.1% disodium edetate, diluted to the mark, shaken, and prepared 1 ml of a reference stock solution containing 50.0 mg of sodium sulfite. Weigh accurately 0.4, 0.6, 1.0, 2.0ml of the reference stock solution, placed in a 10ml volumetric flask, and diluted with a solution containing 0.5% mannitol and 0.1% disodium edetate to form sodium sulfite 2,3 per 1ml. , 5, 10 mg of the control working solution. Accurately weigh 1g of sodium chloride and 1g of solid paraffin (melting point 52~54°C) in a 20ml headspace sample bottle, accurately add 2mL of 2mol/L hydrochloric acid solution, and place the headspace bottle in a 60°C water bath until solid. After the paraffin is completely dissolved, it is taken out and allowed to cool to room temperature to solidify and seal the paraffin wax on the acid layer (if necessary, the acid mist condensed on the bottle wall is blown off by air, and the above 2, 3, 5, 10 mg/ml are precisely measured. 100 μl of each of the control working solutions was placed over the paraffin layer and sealed. Preparation of the test solution: Weigh accurately 1 g of sodium chloride and 1 g of solid paraffin (melting point 52-54 ° C) in a 20 ml headspace sample bottle, precisely add 2mL / L hydrochloric acid solution 2mL, set the headspace bottle In a 60 ° C water bath, after the solid paraffin is completely dissolved, remove it, let it cool to room temperature to re-solidify the paraffin wax, take about 0.2 g of the sample fine powder, accurately weigh it, place it on top of the paraffin layer, and seal it. Linearity and results Enter blank, standard working solution (2mg/ml) and sample. The obtained chromatogram is shown in Figure 1. The above standard solution was injected sequentially from low to high to obtain a standard curve of sulfur dioxide, as shown in Fig. 2. The sample result was substituted into the standard curve, and the obtained result was multiplied by 0.5079, that is, the content of sulfur dioxide in the Chinese medicinal material was obtained. According to the results of Fig. 1, it was found that sulfur dioxide was not detected in the cypress. In this experiment, the RSH three-in-one automatic input is adopted. The sample is because if the conventional valve injection headspace is used to enter sulfur dioxide, since the sulfur dioxide itself has strong reducibility, the reaction may occur during gas transmission, resulting in inaccurate detection results, and the use of gas-tight needles This method does not have this problem. in conclusion This experiment refers to the third method of the determination method of sulfur dioxide residue in the 2015 edition of the Chinese Pharmacopoeia, and follows the pharmacopoeia method to reproduce the detection method of sulfur dioxide for reference. references [1] Brancn AF, etal. Antim icrobials in Foods. M arcel Dckker Inc, 1985:191 Zhejiang Industrial Group Co., Ltd. , https://www.xingyeseafood.com
RSH 3-in-1 Autosampler (Thermo Scientific)
Thermostatic water bath (Thermo Scientific)
Syringe temperature: 105 ° C
Column: GS-GASPRO (30 m × 0.32 mm);
Column temperature: 50 ° C (0 min), 15 ° C / min to 2000 ° C (2 min);
Injection mode: split injection, split ratio 5: 1;
Inlet temperature: 150 ° C;
Carrier gas: nitrogen (99.999%), constant current mode, 2.0 mL/min;
TCD detector temperature: 250 ° C, reference gas flow rate: 1.0 mL / min, negative polarity detection 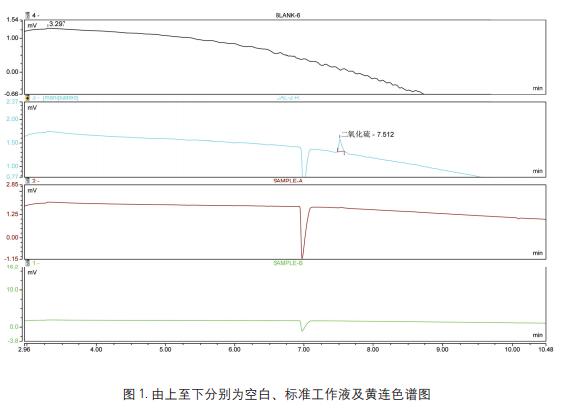
[2] Wang Zhaoji, Guan Xiyao, Wang Jie, Chen Jianxing. Determination of sulfur dioxide in Chinese herbal medicines, Chinese herbal medicine, 2000, 31(2): 97-99