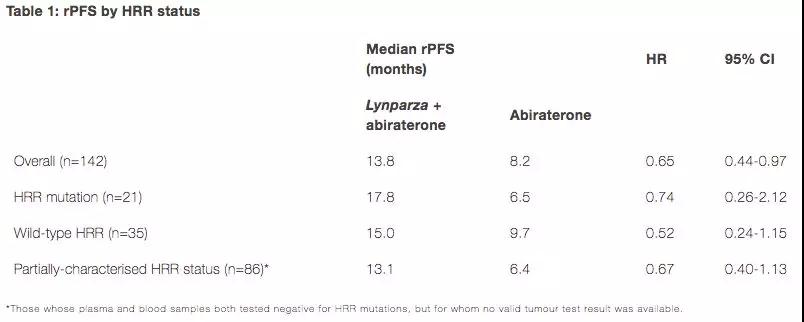
PARP inhibitors overcome advanced prostate cancer, significantly prolonging PFS June 06, 2018 Source: WuXi PharmaTech Recently, AstraZeneca and Merck (MSD) announced at the ASCO annual meeting their data on the development of a new drug, Lynparza (olaparib), for the treatment of metastatic castration-resistant prostate cancer patients (mCRPC). The study showed that Lynparza combined with abiraterone significantly improved median radiographic progression-free survival (rPFS) in patients with mCRPC compared with standard treatment with abiraterone monotherapy. The study was selected for the "Best of ASCO presentation" and published online in "Lancet Oncology." Prostate cancer is the second most common cancer in men. In 2015, there were approximately 1.6 million new diagnoses worldwide and the mortality rate was high. Prostate cancer is often driven by androgen, including testosterone. In mCRPC, although patients use androgen-deprivation therapy to block the effects of androgens, prostate cancer still grows and spreads to other parts of the body. Approximately 10-20% of patients with advanced prostate cancer develop CRPC within 5 years, and at least 84% of them have metastasized when CRPC is diagnosed. Of those men who did not metastasize when diagnosed with CRPC, 33% of patients may have metastases within 2 years. Although the treatment for this type of patient is increasing, their five-year survival rate is only 28%. There is still huge medical demand in this area that has not been met. Lynparza is a first-in-class PARP inhibitor developed by AstraZeneca and Merck. It is also the first target with the potential to exploit DNA damage response (DDR) pathway defects (such as BRCA mutations) to preferentially kill cancer cells. To the therapy. In vitro studies have shown that Lyparza-induced cytotoxicity may involve inhibition of PARP enzyme activity, and increased formation of PARP-DNA complexes, leading to DNA damage and cancer cell death. Lynparza is currently approved for advanced ovarian and metastatic breast cancer, and AstraZeneca and Merck are working to apply it to more cancer types, including prostate and pancreatic cancer. The study, Study 08, is a randomized, double-blind, multicenter, phase 2 clinical trial comparing Lynparza plus abiraterone combination therapy (n = 71) with abiraterone monotherapy (n = 71). Efficacy in treated mCRPC patients, regardless of the status of homologous recombination repair (HRR) mutations. The primary endpoint of the study was rPFS. Secondary endpoints included time to second progress or death (PFS2), overall survival (OS), and health-related quality of life. The median rPFS was 13.8 months in the Lynparza plus abiraterone combination therapy group and 8.2 months in the abiraterone monotherapy group (HR 0.65; 95% CI 0.44-0.97; p = 0.034). The median PFS2 was 23.3 months and 18.5 months, respectively (HR 0.79; 95% CI 0.51-1.21). The median OS was 22.7 months and 20.9 months (HR 0.91; 95% CI 0.60-1.38). A pre-specified exploratory subgroup analysis showed that the patient's rPFS improved regardless of the HRR status (below). â–²The research data (Source: AstraZeneca official website) In addition, the safety of Lynparza plus abiraterone combination therapy is controllable, and there is no adverse effect on the quality of life of patients compared with abiraterone alone. Dr. Noel Clarke, Professor of Urology Oncology, Christie NHS Foundation Trust, UK, said: "This is the first time we have seen the use of PARP inhibitors plus abiraterone to improve patients with metastatic castration-resistant prostate cancer, and this improvement and HRR status Nothing. The data suggests that this combination of treatments may be a promising new treatment for this invasive disease." Dr. Sean Bohen, Executive Vice President and Chief Medical Officer of AstraZeneca Global Drug Development, said: "A previous trial showed that Lynparza monotherapy can improve the remission rate of patients with metastatic castration resistance to HRR mutations. This Study 08 The data suggest that patients with metastatic castration-resistant prostate cancer may benefit from the combination of Lynparza plus abiraterone regardless of the state of HRR mutation." Dr. Roy Baynes, senior vice president and head of global clinical development at Merck Research Laboratories, said: "Transfer castration-resistant prostate cancer patients are at high risk for limited treatment options, and there is still huge unmet medical needs. Lynparza is the first A PARP inhibitor that demonstrates activity in combination with standard therapies in prostate cancer. These data from Study 08 represent another important milestone in the clinical development of Lynparza." Reference materials: [1] Lynparza in combination with abiraterone delayed disease progression in metastatic castration-resistant prostate cancer [2] AstraZeneca official website Insulin Syringes Needle,Disable Syringe,Monoject Syringe,10 Ml Syringe FOSHAN PHARMA CO., LTD. , https://www.full-pharma.com