Single-use Virus Sampling Tubes Virus Sampling Tubes,Blood Specimen Tubes,Serum Collection Tubes,Sample Collection Tubes Henan Anbang Medical Supplies Co., Ltd. , https://www.anbangmedical.com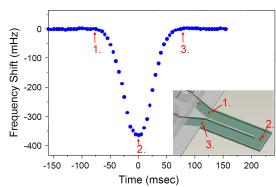
Application of Resonance Quality Test Method in Biopharmaceutical Development
Application of Resonance Quality Test Method in Biopharmaceutical Development
Author: Dr. Lisa Newey-Keane, Malvern Instruments biopharmaceutical product manager
Predicting and measuring protein aggregation is a major challenge in biopharmaceutical formulations. Dr. Lisa Newey-Keane described a new analytical method that facilitates the study of protein aggregation.
Because of the growing share of biomolecular research in overall drug development expenditures, analytical testing has received widespread attention in the rapidly growing biopharmaceutical industry. These molecular developments are not only costly but also subject to strict regulation, so there is an urgent need for more suitable and more rigorous analytical measurement methods. Many people in the industry have mentioned that analyzing bottlenecks is a very worrying issue that may limit the development of drugs.
Unlike small molecule drugs, protein preparations are non-synthetic or non-crystalline and are not uniform in nature. Because of this, it is much more complicated to judge the purity and efficacy of biological therapy than for non-biological molecules. For example, there are many sources of impurities, including aggregates, misfolded conformations, or biomolecules that exist in fully denatured structures. Therefore, the analytical techniques required to ensure and control quality and provide the necessary data for pre-formulation and formulation are quite different from those for small molecule drugs in the pharmaceutical industry. This complexity and variability not only gives manufacturers, but also brings many new challenges to regulators.
The selection of suitable candidate molecules involves various physicochemical testing procedures in other analyses to exclude those molecules that are likely to become downstream "problem molecules." One of the most important questions to answer is how these molecules will behave in the formula. An important area of ​​concern is protein aggregates, as well as the immune response that may be triggered by aggregation. Regulators have clearly expressed concerns about the potential immunogenicity of protein aggregates ranging from 0.2 microns to 2 microns in diameter, but existing particle size determination techniques are not sufficient to provide quantitative data for this range. They let you know that particles with this size exist, but you can't determine the amount that exists. The Resonance Mass Measurement (RMM) technique applied to the Malvern Archimedes system compensates for this deficiency because it can not only measure particle size, but more importantly, it can count particles from 50 nm to 5 microns in diameter. .
Technical introduction
Resonance mass measurement relies primarily on a mechanical resonance structure that can detect mass changes. An increase or decrease in mass can cause the resonant frequency of the structure to rise or fall. This provides a basis for measurement quality due to the very accurate measurement of the frequency. In order to measure the mass of tiny particles suspended on the liquid, the resonator has a built-in microfluidic channel. As the suspended particles pass through the structure, it changes the overall mass of the resonator, thereby changing its resonant frequency. As shown in Figure 1, the particles enter the resonator at position 1. When the particles reach position 2 at the end of the resonator, the offset to the resonant frequency reaches a maximum. When the particles exit at position 3, the resonant frequency returns to the baseline.
Measurement of particle mass can be achieved by measuring the offset of the resonant frequency from the baseline. For particles with buoyancy, such as oil droplets (see below), the opposite effect can be observed and the resonant frequency will be positively offset relative to the baseline.
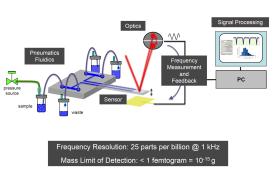
Resonance mass spectrometry is used to measure very small particles, requiring the use of a resonator of very small mass. In the Malvern Archimedes system, MEMS (Micro Electro Mechanical Systems) sensors can meet this requirement. Each sensor chip consists of a microfluidic network and a tiny cantilever that resonates at a specific frequency. A microfluidic channel is built into the cantilever. The resonant frequency of the cantilever changes as the fluid system in the instrument pushes the sample through the channel. The change in resonant frequency is measured by laser, first focused to the top of the cantilever and then sent to a separate photodiode detector.
Each particle passing through the sensor causes a change in the resonant frequency, resulting in an accurate measurement of the buoyancy quality of the individual particles in the sample, regardless of whether the data is negative. Through such measurements, the mass, particle size (equivalent sphere), and surface area of ​​the particles can be calculated. The overall measurement of sample concentration, density, volume and polydispersity is also possible.
Quantitative measurement of protein aggregates
Initially, protein aggregates were at the dimer level, after which the diameter climbed all the way to tens of microns, and aggregates above the upper range were typically measured using a flow microscope. Resonance mass measurements can be applied in areas below the scope of flow microscopy, including those with sub-micron to a few microns in diameter, and those that are not easily evaluated by other methods for quantitative evaluation. This is also where the immunogenicity is affected, and where new regulatory requirements are of concern.
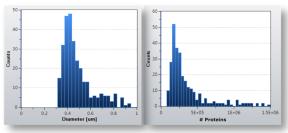
Figure 3 (a) shows the particle size distribution of submicron IgG protein aggregates in 4 μL of formulation buffer.
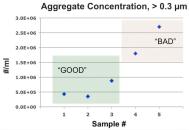
The concentration of aggregates having a particle diameter of more than 300 nm was 4 × 10 6 per ml as measured by RMM. Since the measurements are mass based, the particle size distribution can be expressed as the number of protein molecules (known masses) that form each aggregate (Fig. 3(b)). Shown in Figure 3(c) is the measurement of the protein sample after increasing the shear stress for a period of time, and shows that the concentration of aggregates increases from 300 nm to 1 μm throughout the experiment. Aggregates of this size show a 10-fold increase, roughly corresponding to an aggregate cascade, and the concentration of submicron aggregates is associated with poor quality of the formulation under pressure.
Detection of pollutants
Another popular subject in the analysis of protein preparations is silicone oil, which is commonly used as a lubricant in syringes and containers containing the formulation. Silicone oil is often mixed into the formulation to form oil droplets that are close in size to the aggregate. But the main problem is not biocompatibility, as silicone oil droplets are generally considered safe. The bigger problem is that in some measurement methods, oil droplets are mistaken for protein aggregates and therefore may affect the accuracy of the results.
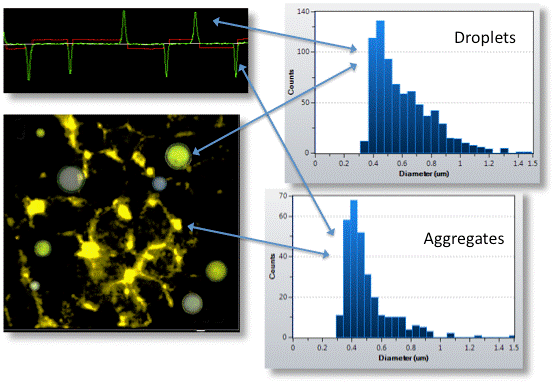
RMM can distinguish silicone oil droplets from protein aggregates by buoyancy measurement. For example, in Figure 4, aggregates with a density greater than the suspension buffer are represented by negative peaks in the frequency trajectory.
Silicone oil droplets are generally denser than buffers and form positive peaks in the frequency trajectory because their presence reduces the overall mass of the sensor and increases the resonant frequency of the sensor. Each group provides a separate distribution.
Today's technology
The key reason for the success and application of RMM technology in the use of conventional analytical instruments is that it solves the problem of critical particle size ranges in protein aggregation measurements. Equally important, when processing small doses of valuable materials, only 100 μL of sample with a viscosity of 100 cP is required and measurements can be taken without sample preparation.
Resonance mass spectrometry is an attractive solution for protein characterization due to the ability to detect and accurately count particles in the 50 nm to 5 micron size range and to reliably measure buoyancy mass, dry mass and size. Quantitative information about formulation and stability can be translated into information on performance and its stability. At present, RMM has been used in the early development stage of biologics to detect and quantify aggregates, which realizes the concept of “the earlier the discovery, the lower the costâ€.
Malvern's new bioscience development program
In the traditional mode, it takes time to bring an analytical instrument to market and make it a perfect end product. But this model is completely unsuitable for the biopharmaceutical sector because it often only answers the question of transition. Researchers at the forefront of biopharmaceutical research need analytical tools that solve current problems; the challenge for suppliers is the need to predict unknown areas in which rapidly changing analysis and regulatory requirements continually test relevant participation. By. It is therefore necessary to understand what is measurable, what measurement results can provide valuable predictive information on quality, and anticipate the measurements needed in the future.
Malvern has helped the rapid development of the bioscience industry by creating bioscience development programs. The program is designed to keep pace with the rapid development of biopharmaceutical technology and to take responsibility as an analytical instrument manufacturer to quickly deliver compelling solutions to rapidly evolving analytical and regulatory challenges. The key to success is to develop high-level technology development cooperation with a highly confident and open attitude, and to establish flexible technology and product development processes to maintain absolute focus on the identified analytical needs.
Although the Bioscience Development Program is an integral part of the Malvern organization, its operations are carried out in factories near Washington, DC. The program is led by Dr. E Neil Lewis, Chief Technology Officer of Malvern, where he leads a team of scientists, engineers and business developers. These members, although located everywhere, are closely linked to the Malvern core team. They focus on the requirements of the biopharmaceutical industry, especially the biochemical and biophysical characterization of formulations and product development.
This fast-moving, well-funded and targeted program will drive close collaboration between biopharmaceutical companies, technology innovators and leading academic institutions. By establishing this mutually beneficial relationship, Malvern can understand the requirements of emerging industries in real time and respond quickly, and the biopharmaceutical sector can intervene as early as possible and help design the cutting-edge technologies it needs. The first result of the collaboration was the agreement to bring the Malvern Archimedes system to market. With the further development of technology, this year there will be a fully mature product launch.
For further information on the Malvern Bioscience Development Program, please contact Dr. E. Neil Lewis
Neil.
[ image ]
Figure 1: Measuring particle mass in a fluid
Figure 2: Configuration of a resonance mass spectrometer (Malvin Archimedes system)
Figure 3: (from left to right) (a), (b) and (c): Aggregate detection using the Malvern Archimedes system
Figure 4: Demonstration of the ability to distinguish between protein aggregates and contaminated silicone oil droplets