Cell wall components such as Gram-negative bacteria such as E. coli and Pseudomonads are considered endotoxin. They have a hydrophilic polysaccharide and a lipophilic lipid structure, which are different from the bacteria from which they are derived, and have high thermal stability and pH stability. Endotoxins are a source of heat that can cause fever if they come into contact with the mucous membranes or if they enter the bloodstream (see Steck, 20061 for details). According to the general pharmacopoeia, the endotoxin content in the pharmaceutical production process must not exceed the specified limits. The emergence of endotoxin may lead to death in mammalian cell cultures required for the production of biopharmaceuticals (eg, immunoglobulins). Therefore, ultra-pure media is required for biopharmaceutical production, cell line passage, or cell culture, and of course ultra-pure water is required. The endotoxin levels contained in them have been proved to be lower than the specified limits. . The purpose of this study was to show that the ultrapure water produced by the Arium pro VF system has endotoxin levels far below the specified limits and can be used in these applications. experimental method In order to test the endotoxin content in the ultrapure water produced by the Arium pro VF system, we used the gel method and the color method to test.  Experimental result In the concentration range of 0.001 EU/ml-0.05 EU/ml, the gel method showed a negative result, meaning no gel formation (no endotoxin detected; see Table 1); when the concentration was ≥0.1 EU/ml An agglutination reaction has occurred. This result shows that endotoxin does not appear in the ultrapure water produced by the Arium pro VF system within the detection limit of the gel method. In the chromogenic method, a 60 adsorption reading at a wavelength of 405 nm (see the figure below) produces a curve of different slopes depending on the endotoxin content in the standard sample. The time required to reach the absorption rate is calculated by a special computer program and used as a basis for inferring samples with unknown endotoxin content (see Table 1 - Color Rendering Test for specific values). Since the standard sample with the lowest concentration (0.005-0.001 EU/ml) and Arium water did not reach the desired extinction value for the calculated endotoxin content, they were considered to be below the detection limit. The value obtained with Arium pro VF ultrapure water is significantly lower than the plotted point of the 0.001 EU/ml curve. The graph below shows the data set of endotoxin concentrations measured in tap water, deionized water (DI water) and Arium water compared to two selected endotoxin standards (0.05 EU/ml and 0.005 EU/ml). . The endotoxin content in the water sample is calculated by using the value measured by the endotoxin standard, as shown in Table 2. In the DI water sample, endotoxin was detected at 0.02 EU/ml, which is lower than the current effective value of WFI (water for injection). An endotoxin load of up to 25 EU/ml was unexpectedly detected in tap water. The results were not further analyzed. In contrast, unexpectedly low values ​​were detected in ultrapure water produced by the system, < 0.001 EU/ml, which is well below the usual limits. In the DI water sample, endotoxin was detected at 0.02 EU/ml, which is lower than the current effective value of WFI (water for injection). An endotoxin load of up to 25 EU/ml was unexpectedly detected in tap water. The results were not further analyzed. In contrast, unexpectedly low values ​​were detected in ultrapure water produced by the system, < 0.001 EU/ml, which is well below the usual limits. in conclusion The test results show that the ultra-pure water produced by the Arium pro VF system can be used as a convenient and affordable option for sample preparation for endotoxin testing because the endotoxin concentration detected in the ultrapure water produced is very low. (<0.001 EU/ml). The results obtained also confirmed early experiments on the discovery of endotoxin loading <0.001 EU/ml in Arium ultrapure water. The endotoxin concentration in this ultrapure water is much lower than the limits set by the United States Pharmacopoeia, which makes the ultrapure water theoretically applicable to the production/endotoxin monitoring of pharmaceutical products. Related examples include product formulations, diafiltration solutions, chromatography buffers, and water for extraction, washing, aseptic steps, and cell culture protocols. In the context of cell culture applications, we need to be highly vigilant about contaminants at all stages of the process. In order to maintain control over the effects of endotoxin on cell culture and cell sensitivity, especially when associated with such endotoxin, the medium used for cell culture must be clearly free of detectable endotoxin content (see Reference 5). Sartorius arium® series ultra-pure water machine - the achievement of your "great gift" activity is in full swing! Download the full-text view of the experimental design: Download the full text References / More Information 1 Steck, S. : Endotoxine und ihre Bedeutung bei R & D Applikationen. Bioforum 6/2006. Acknowledgement We would like to express our special thanks to Dr. Stephanie Steck of Stephanie Steck of Rentschler Biotech¬nologie in Laupheim, Germany, for his busy schedule to review this article and to have a constructive discussion on the subject. Evaporative Cooler Air Conditioners,Portable Air Conditioners,Air Coolers from Aircon Direct,Air Cooler with Water Ningbo Shuangtuo International Trade Co., Ltd. , https://www.nbst-sports.com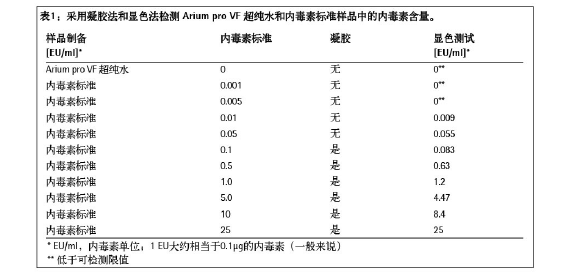
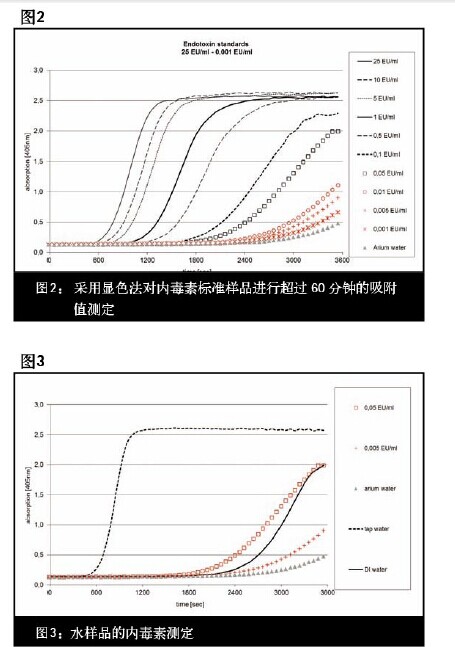
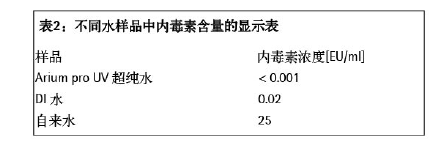
2 Endosafe® -PTSTM Glucan Assay, Charles River Laboratories International, Inc, 2009.
3 Endotoxin Compendium, V 2.11, Hyglos GmbH, Bernried, Germany.
4 Untersuchungsbericht Fresenius Medical Care, Deutschland GmbH, Werk St. Wendel, 2007.
5 Dawson, ME : LAL Update von Associates of Cape Cod Incorporated, March 1998, Vol. 16, No. 1. Adapted from the original German article by Dr. Herbig, June 7, 2012