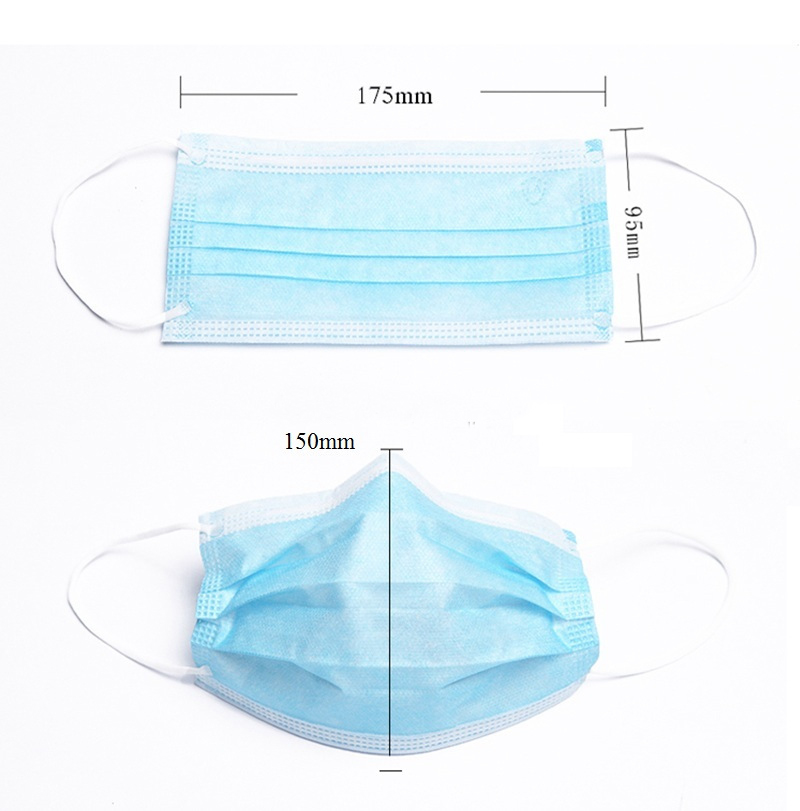
face mask used for daily protection, with 3 ply non-woven fabric, It can effectively filter droplets and block direct contact between virus and human body. The fabric is breathable and comfortable,it won't cause skin allergy.
We are professional manufacture of medical equipment with 6 years experiance.
The company conscientiously implements the "Regulations on the supervision and administration of medical devices" and other laws and regulations, and adopts a scientific management mode. The company has successively passed the EU CE certification and CFDA "medical device production quality management specification" assessment.
Adhering to the concept of "quality first, integrity-based, continuous change and long-term commitment", the company is committed to providing customers with high-quality, high safety performance products and high-quality services.
Disposible Face Mask,Face Mask With Logo,Face Mask With Designs,Disposable Medical Face Mask Shanghai Rocatti Biotechnology Co.,Ltd , https://www.ljdmedicals.com
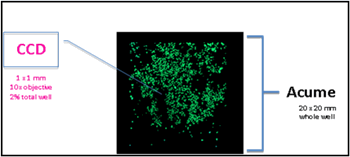
Acumen's unique full-hole scanning field of view can obtain an image of 20X20mm area in a single time, which is equivalent to the area of ​​one hole of a 24-well plate. The advantages are as shown on the right, and most of them solve the drug sensitivity using primary tumor cells. Technical problems detected.



Translational Medicine Research: Exploring pharmacological effects and individualized treatment plans using primary cell models
How to apply the findings and methods of basic research to the guidance and monitoring of clinical disease treatment is also one of the important directions of translational medicine. The primary killer of human health--the important treatment for cancer is chemotherapy. For a long time, because of the inability to predict the sensitivity and drug resistance of different individuals before chemotherapy, it is mainly based on the experience of clinicians. Administration, with great blindness. In many cases, this unscientific administration and treatment not only causes the suffering of the patient, but also induces the combined resistance of the tumor cells to various drugs, leading to the failure of chemotherapy. Therefore, translational medicine requires a detection technology that can screen sensitive drugs for patients before chemotherapy, objectively select and optimize chemotherapy drugs and their combinations for different individuals, and achieve case-based treatment of cancer patients.
At present, the drug sensitivity monitoring technology for in vitro tumor cells is mainly to collect the primary cells obtained by collecting and separating the tissues and body fluids, and to observe the direct effects of the drugs on tumor cells (including the efficacy). And toxicity), according to the difference in the inhibition rate of cultured cell growth according to the concentration of chemotherapeutic drugs, computer software analysis and reference to the corresponding judgment indicators, thereby screening effective chemotherapy drugs and their combination drugs for different individuals, for personalized treatment.
At present, there are several major bottlenecks in the existing in vitro susceptibility test methods at the technical level:
a) The need for more tumor tissue, affecting the needs of other pathological examinations for tumor specimens;
b) The components in the tumor tissue are complex, the heterogeneous cells interfere with the target cells, and the primary cells are not easily purified;
c) The primary tumor cells are difficult to culture, and it is impossible to obtain enough cells for evaluation of multiple chemotherapeutic drugs and drug susceptibility at multiple concentrations;
d) that the manipulation and detection process is complex and destroys the integrity of the primary cells;
e) that imaging studies rely on a wealth of cytological experience and are susceptible to human factors; and imaging-dependent assays are unable to perform an overall assessment of drug response by a large number of population cells due to the size of the field of view;
f) does not have good repeatability of results;
g) low compliance rates in and out of the body;
Accelerate the process of personalized medicine with high efficiency, low cost, and high quality data
â— Full-hole scanning, obtaining a large amount of data and comprehensive, reducing manual error and sampling error, overcoming the problem of cell inhomogeneity in the hole caused by cell death caused by drug killing, and improving the repeatability of the result;
â— Detection is less dependent on the experience of the researcher and more objective;
â— Reduced the need for sample size;
â— Assess the sensitivity and tolerance of the population cells as a whole to the drug;
â— For primary cells that are particularly difficult to separate, purify and expand, single primary cells can be collected for more drug and concentration gradient drug sensitivity tests, making the experimental data more timely, complete, and clinically relevant;
â— Due to the reduced demand for primary cells, it can prevent the variation of primary cells caused by excessive amplification.
Acumen eX3 laser scanning technology is from TTPLabtech, a subsidiary of TTPGroup, Europe's largest independent technology development and innovation group. In keeping with the corporate philosophy of building long-term partnerships, TTPGroup has created and developed the most renowned R&D outsourcing service company, The Technology Partnership Plc., in the beautiful technology park near Melbourn Science Park in Cambridge, England, which has fostered a number of global influences. Innovative companies, including TTPCom (London's main board in 2000 - followed by Motorola's overall acquisition), TAP, TTPLabtech, etc., the group has repeatedly won the high-profile Queens Award. TTP Labtech is a wholly owned subsidiary of TTPGroup, specializing in the development, production and sale of innovative precision instrumentation and equipment for life science research and development. Since entering the Chinese market in 2007, its full line of products has been well developed in the Chinese market, and its reputation and popularity have gradually increased. The team has also gained recognition from customers with dedicated and professional services. Based on the confidence in the continued development of the Chinese market, and firmly believe that China's production, research and development resources and TTPGroup's innovative capabilities are greatly complementary.
Acumen eX3 is the latest generation of ultra-high-speed multi-functional cell analysis instrument introduced by TTPLabtech since 2001. It is widely used in cell-based HTS Biology and high-content screening (HCS) for drug development. , and mechanism research in the field of translational medicine and cell model susceptibility testing. Its high-throughput biology applications are widely used in world-class R&D units such as NIH and national oncology research institutes, and are among the most cutting-edge and innovative cytology platforms in translational medicine. Multinational pharmaceutical companies and CRO customers: AstraZeneca, Lilly, Sino-US Crown, Hutchison Whampoa, etc.; some scientific research users: Academy of Military Medical Sciences, China Pharmaceutical University.