Cooling Agent, is a general term for all the chemicals that can produce cooling effects and are not strong. The most common Cooling Agent is menthol (especially L-menthol), but it should not be used in large quantities due to its strong odor and strong irritation to the skin, mucous tissue, and eyes. Therefore, many scientists have synthesized and extracted a new generation of cooling agent. WS-23,flavor,Menthol,WS-5,Koolada,WS-12 Xi'an Double H Health Technology Co., Ltd , https://www.dhextract.com


High sensitivity nitrosamine analysis by gas chromatography-mass spectrometry/mass spectrometry
High sensitivity nitrosamine analysis by gas chromatography - mass spectrometry / mass spectrometry
Alex Chen1, Hans-Joachim Huebschmann2, Li Fangyan3, Chew Yai Foong3 and Chan Sheot Harn3
1Alpha Analytical Pte. Ltd., Singapore; 2Thermo Fisher Scientific, Singapore, 3Health Sciences
Authority, HSA Singapore
Key words
Nitrosamine, food safety, beer, TSQ 8000, gas chromatography-mass spectrometry/mass spectrometry, quantitative analysis, confirmation,
Automatic SRM, TraceFinder
Introduction
Nitrosamines are common names for compounds such as N-nitrosoalkylamines. There are many such compounds known to contain different alkyl groups. The simplest N-nitrosoalkylamine contains two methyl groups, N-nitrosodimethylamine (NDMA). Nitrosamines are common highly toxic compounds that are highly carcinogenic to humans and animals, and high doses can cause severe liver damage and internal bleeding.
Nitrosamines in food are mainly produced by nitrous acid. Nitrous acid is usually added as a preservative to meat and meat products to avoid poisoning caused by Botox. Antioxidant additives such as vitamins can inhibit the conversion of nitrous acid to nitrosamines. Another source of nitrosamines is produced by the reaction of nitrogen oxides with alkaloids, which has been reported in the process of drying germinated malt during beer production. Since the level of nitrosamines in malt and beer has been greatly reduced during the fermentation process, better analytical performance is required to perform this testing task. In addition to the routine control items of other daily foods, low-dose nitrosamine detection of malt in beer is also necessary.
The "classical" nitrosamine detection method that has been used for many years is analyzed by gas chromatography using a tandem thermal energy analyzer (TEA) detector. The special TEA detector is chosen because the detector can generate NO from nitrosamines, and NO can perform specific chemiluminescence reaction with ozone to achieve specific nitrosamine detection. Today, as the sensitivity requirements for detection methods continue to increase, TEA's detection limits and their complex operating procedures are no longer sufficient to meet current low detection limits and high sample throughput requirements. Mass spectrometers are constantly replacing TEA.
The EPA Method 521, established by Munch and Bassett in 2004, provides a GC/MS method based on chemical ionization (CI) and ion trap mass spectrometry with internal ionization, rather than the standard band. Quadrupole or ion trap mass spectrometer with external ion source. Now with the development of technology, GC-MS triple quadrupole can also provide high sensitivity and high selectivity analysis in low molecular weight regions, enabling very low concentrations of nitrosamine detection, even in low concentration detection in complex samples. ,become possible. This possibility stems from the use of simpler, standard techniques using conventional electron bombardment sources (EI) to establish a convenient method for low-concentration nitrosamine detection.
This application note describes a complete set of methods for routine detection and quantification of nitrosamines in food using GC-MS/MS. The food matrix in this work includes a variety of different malt beer products as well as the beer itself sold as a final food product. During the development of the method, we pay special attention to optimization to provide a rapid and easy to implement routine detection method while achieving the high sensitivity required for the detection of nitrosamine compounds.
The sample processing method is based on the AOAC Official Method (2000), 982.11 with minor modifications. We have established a solid phase extraction method using a Celite diatomite column and eluting with DCM to separate nitrosamines from beer samples.
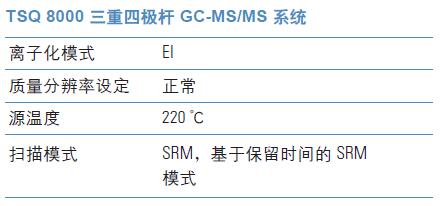
Experimental condition
GC-MS/MS instrument
SRM method establishment
We used the AutoSRM software from the Thermo Scientific TSQTM 8000 GC-MS/MS software suite to create a triple quadrupole mass spectrometry method and did not make any manual modifications to the AutoSRM generated method. An autosampler vial containing a standard solution of the nitrosamine compound to be analyzed is intended for use in the AutoSRM program.
The AutoSRM program automatically performs the following three steps:
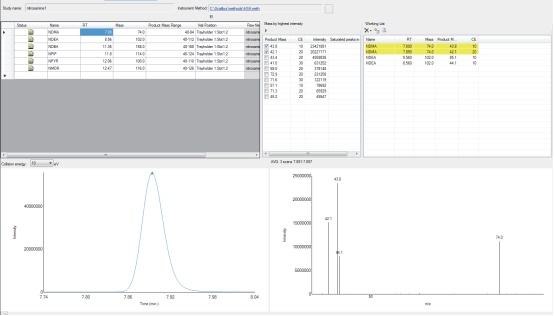
1. First perform a full scan analysis of the standard solution (Figure 1.). The strongest ion from the full sweep will be used as the primary ion.
2. Perform the secondary ion (child ion) spectrum acquisition of the primary ion (parent ion) determined in the previous step (the number of primary ions can be set according to the analysis requirements). Find the strongest secondary ion generated by each primary ion (you can manually select the most interesting primary ion to proceed
One step optimization).
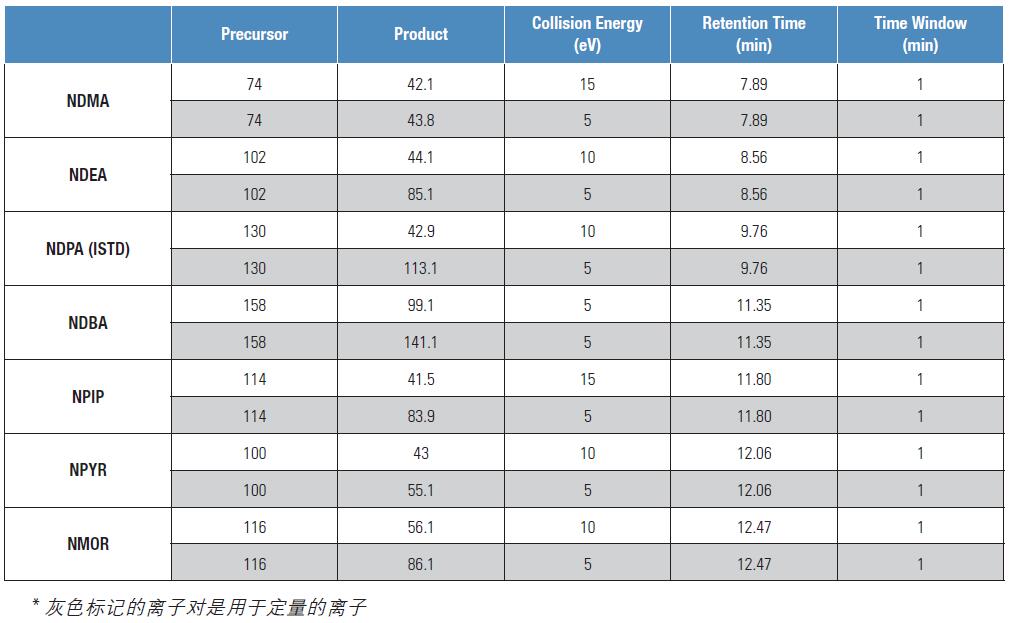
Table 1. SRM method settings generated by AutoSRM
3. Optimize the collision energy for selected parent ion/ion ion pairs for all compounds to achieve maximum compound response and best method sensitivity (Figure 2).
The AutoSRM program can be started from a standard vial as needed to complete the required number of injections.
Table 1 is the SRM ion pair table automatically generated by AutoSRM. The table also shows the SRM acquisition method for the TSQ8000 GC-MS/MS in Timed-SRM mode with a 60 second short acquisition window around the compound elution time. There is no need to make any other settings for the scan period, or if it needs to be monitored outside of the elution time of a compound, the compound needs to be added manually.
Sample determination
Among a wide variety of possible nitrosamine compounds, the process encompasses those nitrosamine compounds that are reported to be associated with the process of germination of malt. The samples analyzed included a malt beer sample to which no standard was added, and 4% ethanol as a blank. For analysis of other food matrices, other compounds can be added to the method at any time by following the steps established by the AutoSRM method described above.
Experimental result
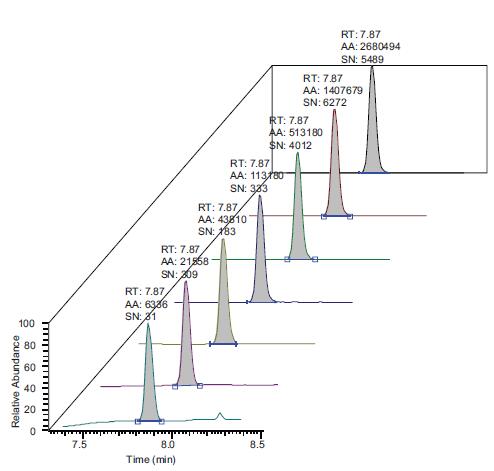
The chromatogram of the nitrosamines contained in this method exhibits a faster outflow, from NDMA of 7.87 to 12.47, enabling shorter cycle times and increased sample throughput. Figure 3 shows the peak intensities obtained with the lowest concentration of -1 ppb in the calibration curve. It can be seen from the figure that the signal to noise ratio of NDMA detection is still very good.
Figure 1. AutoSRM for NDMA from the EI full-sweep spectrum for primary ion selection
Figure 2. Collision optimization of all nitrosamine primary ions by AutoSRM
Figure 3. Chromatogram of a standard mixture at a concentration of 1 ppb
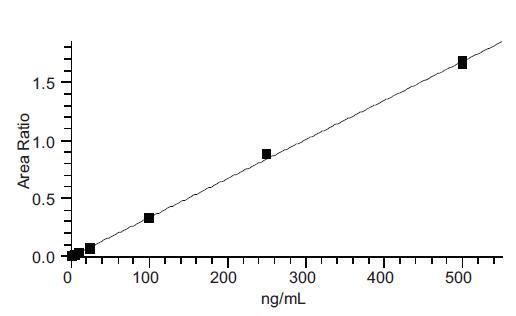
We performed quantitative calibrations over a wide range of concentrations from 1 ppb to 500 ppb. Figure 4 shows the nitrosamine peaks obtained from all calibration tests. In all cases, the peak shape of the NDMA is completely symmetrical, without tailing, and a very reliable peak area integral value is obtained without any manual correction. Figure 5 shows the NDMA calibration curve for sample quantification. The curve R2 is greater than 0.99 and the linearity is excellent. This TSQ 8000 GCMS/MS method achieves the same level of calibration accuracy for all nitrosamines.
Figure 4. Calibration curve measurement results for NDMA from 1 ppb (bottom) to 500 ppb (top)
Figure 5. Calibration curve for NMDA from 1 ppb to 500 ppb
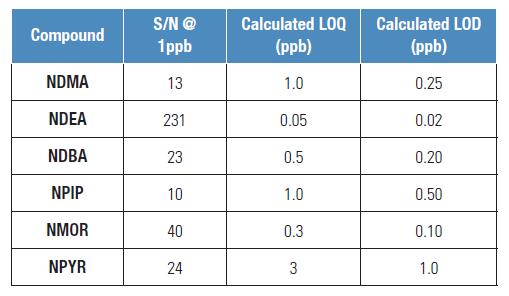
LOQ determination
The calculation of the minimum limit of quantitation (LOQ) and the minimum detection limit (LOD) are based on the signal-to-noise ratio of the chromatographic peak. The LOQ calculation is based on a signal-to-noise ratio of 10 and the LOD is based on a signal-to-noise ratio of 3.
Table 2. Calculation of LOQ and LOD for this method
Result confirmation
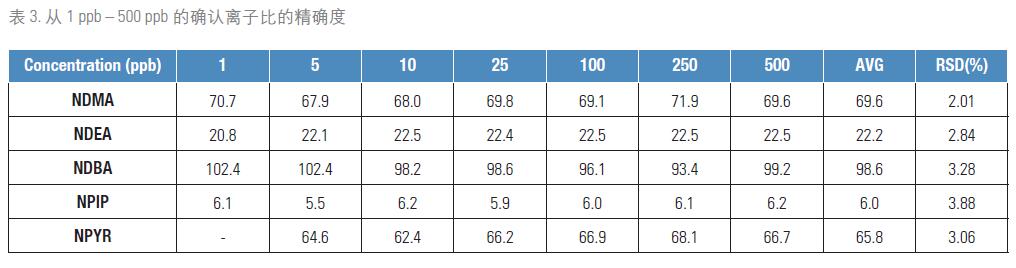
Compound confirmation is achieved by the ion ratio check function provided by Thermo Scientific TraceFinderTM Quantitative Analysis Software, which compares the ionic strength of SRM and qualitative SRM for quantification. Standards covering the 1 ppb to 500 ppb concentration range were subjected to three replicate injection tests and used to calculate the ion ratio accuracy. The results are shown in Table 3. Although all of the ions being detected are in the low mass range and may be subject to multiple disturbances, the accuracy of the ion ratio of the secondary ions is well maintained at 1-4%.
For quality control in sample analysis, positive results are confirmed by ion ratio check when performing quantitative data processing with TraceFinder software. For all compounds, the ratio of ions collected by the two secondary ions should be kept within ± 5% (10%), in accordance with the calibration values ​​obtained from the standard. This provides a solid safety guarantee for routine sample testing. Table 3 lists the average ion ratios of all the nitrosamines involved in this study.
Sample detection
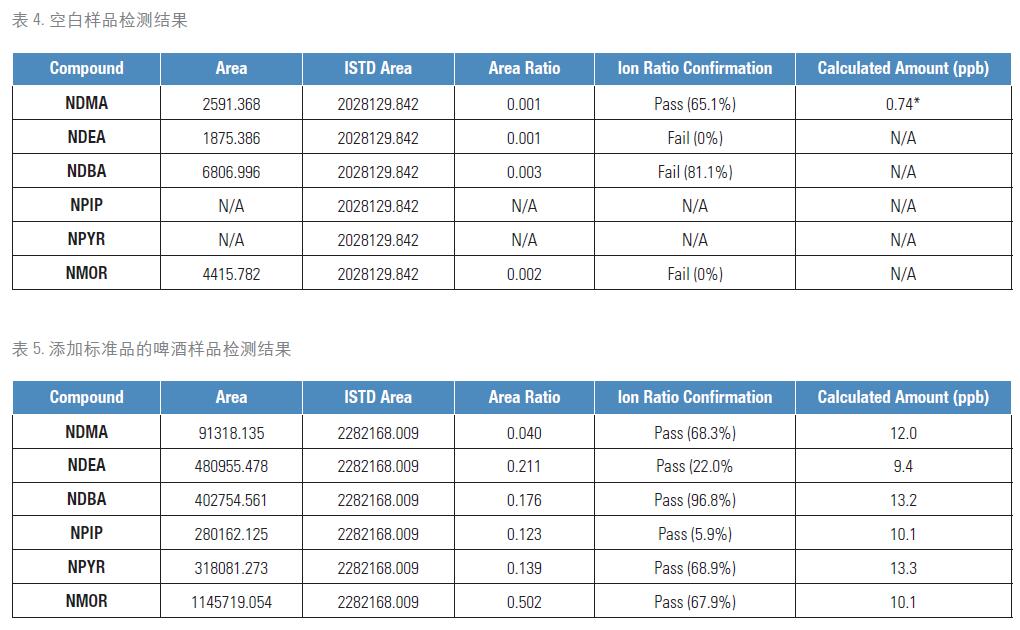
We tested a variety of samples, including blanks and beer samples with standard additions. The test results of the blank sample are shown in Table 4. The low concentration of NDMA detected in this sample was calculated to be below the calibration curve concentration and also below the LOQ. Therefore, at this LOQ, the blank sample can be confirmed to be free of nitrosamine compounds.
Another sample was prepared by adding different concentrations of nitrosamines to the beer. All nitrosamine compounds were detected and quantified in the low concentration range, as shown in Table 5. Each of the peaks used for quantification passed the ion specific mass control, and was able to confirm each compound that was positive by calculating the ion ratio at such a low concentration.
in conclusion
Through the GC-MS/MS method based on the TSQ 8000 system described herein, all nitrosamine compounds involved in the study can be safely detected and accurately quantified at the low concentrations required for food safety control. The LOD of all compounds was less than 1 ppb at the lowest concentration used for the quantitative calibration curve of 1 ppb. The TSQ 8000 GC-MS/MS exhibits a wide linear range and excellent accuracy over the 1-500 ppb range. The linearity of all calibration curves is very good, R2 is greater than 0.99.
The TSQ 8000 GC-MS/MS also shows very good ion specific stability and is suitable for confirmation of positive samples. The ion ratio of all compounds, even at LOQ concentrations, is less than 4% RSD. The use, establishment, and maintenance of nitrosamine detection methods based on GC-MS/MS are simple. Our unique AutoSRM software automatically finds and optimizes SRM ion pairs and collision energy even when analyzing new unknowns.
Based on the GC-MS/MS method shown here, the TSQ 8000 GC-MS/MS accurately and reliably measures nitrosamine levels in real samples.
The GC-MS/MS method described in this article for the determination of nitrosamines in food using TSQ 8000 GC-MS/MS can be used directly for routine food safety control. The standard GC-MS/MS triple quadrupole instrument used in this method is also widely used in other areas of conventional food safety control, such as pesticides, POPs, or polycyclic aromatic hydrocarbons. The method is fast to detect, supports high sample throughput, and provides very high sensitivity and precision. The method uses conventional electron bombardment sources for ionization and achieves low concentrations of nitrosamine quantification. We recommend this method as a high-yield alternative to the aforementioned chemically ionized ion trap method using liquid CI reagents.
references
[1] Robert K. Boyd, Cecilia Basic, Robert A. Bethem, Trace Quantitative Analysis by Mass Spectrometry, 2008 John Wiley & Sons, Ltd.
[2] Material Safety Data Sheet NDMA.
[3] Agency for Toxic Substances & Disease Registry, Public Health Statement for n-nitrosodimethylamine, 1989, http:// cdc.gov/toxprofiles/phs141. html
[4] Richard A. Scanlan, Nitrosamines and Cancer, Linus Pauling institute, http://lpi.oregonstate.edu/f-w00/nitrosamine.html .
[5] Mario M. Mangino and Richard A. Scanlan, N-Nitrosamines in Beer, N-Nitroso Compounds, ACS Symposium Series, Vol. 174,1981, Chapter 17, 229–245.
[6] Munch, JW, Bassett, MV Method 521: Determination of nitrosamines in drinking water by solid phase extraction and capillary column gas chromatography with large volume injection and chemical ionization tandem mass spectrometry (MS/MS) (Version 1.0) US Environmental Protection Agency.
[7] Raymond E. March, Richard J. Hughes, Quadrupole Storage Mass Spectrometry, 2nd Ed., John Wiley & Sons 1989.
[8] AOAC Official Method 982.11, 2000.
[9] Introducing AutoSRM, Thermo Fisher Scientific, Technical Brief No. AB52298, 2012.
[10] Thermo Scientific TSQ 8000 Triple Quadrupole GC-MS/MS Instrument Method, Thermo Fisher Scientific, Technical Brief No. AB52299, 2012.